Chemistry and Chemists № 1 2025
Journal of Chemists-Enthusiasts
| Content | Chemistry experiments - video | Physics experiments - video | Home Page - Chemistry and Chemists |
|
Chemistry and Chemists № 1 2025 Journal of Chemists-Enthusiasts |
Acetone, Acetone Peroxide, and Chloroacetone - pt. 4, 5, 6 Bad Chemist |
|
Having noticed a mistake in the text, allocate it and press Ctrl-Enter
Synthesis of Acetone Peroxide Using Acetone Diluted with Unknown Solvent - part 4
It turned out that the solvent sold under the name 'Acetone +' contains acetone mixed with unknown additives; most likely, acetone was diluted with petroleum products. For the successful synthesis of acetone peroxide, the petroleum products must be separated from the acetone. The most reliable method for this is fractional distillation. While fractional distillation was possible, it required acquiring or finding the necessary equipment, followed by a trial distillation of the mixture to determine which fractions and in what quantities would form.
Синтез перекиси ацетона с использованием ацетона, разбавленного неизвестным растворителем - часть 4 I came up with a simpler method for carrying out the synthesis. First, I measured 30 ml of a 30% hydrogen peroxide solution and 30 ml of the acetone (containing unknown petroleum products) using a cylinder, then mixed the liquids in a beaker. As a result, some of the acetone passed into the aqueous solution (of course not completely), while the petroleum products formed a separate layer. How much acetone was originally in the mixture, and what fraction of the acetone passed into the aqueous solution, remained unknown. However, this uncertainty was preferable to the complexity of performing fractional distillation. I then poured both liquids into a separatory funnel and drained the lower aqueous layer into a beaker. This solution contained hydrogen peroxide and some acetone. I neglected the cooling, added 30 ml of 13.5% hydrochloric acid solution to the mixture, and stirred the liquid. For a moment, the mixture turned orange but quickly became colorless. Under other circumstances, such a color change might have been exciting: two colorless liquids mix, momentarily turning orange before becoming colorless again. However, I was not interested in performing a demonstration since my goal was the synthesis of the compound. The reaction mixture became cloudy, but I suspected this cloudiness was caused not by the formation of the desired solid phase, but by the release of oxygen bubbles. When I touched the beaker, it felt hot. I cooled the mixture with running water and then placed the beaker in a cold water bath. As the release of oxygen bubbles slowed, the solution became clear again. No solid phase had formed. The solution cooled down, so I removed it from the water bath and placed it on the table. While waiting for the solid phase to form, I conducted a couple of experiments with our low-quality acetone, which I will describe in the next part. After a few hours (by the end of the day), I noticed that a white precipitate had formed in the reaction mixture, held near the surface by gas bubbles. This was acetone peroxide. The amount of the precipitate was small. I left the beaker overnight, and by the next morning, the quantity had increased significantly, though it was still small (I visually estimated the mass of the precipitate to be about 0.1-0.2 g). The release of oxygen bubbles continued, causing the acetone peroxide to collect at the top of the beaker. I filtered the precipitate, washed it with distilled water on the filter, and left it to dry at room temperature. Once dry, I took a small amount of the substance, placed it on paper, and ignited it with a flame. A yellow flash occurred, accompanied by a pop. The substance decomposed, leaving no solid residue or marks on the paper. I left the acetone peroxide to continue drying on the filter paper until the next day. When I returned to the lab, I found that most of the acetone peroxide had evaporated from the paper filter. Only a small amount remained - just 0.04 g! I had read that acetone peroxide is volatile, and this volatility presents safety concerns, as the evaporated substance can condense on threads or ground glass joints of containers, forming crystals. These crystals can detonate upon opening the container, triggering an explosion of all mass of the substance in the experimenter's hands. However, I had not anticipated how quickly acetone peroxide could evaporate. Later, I found a statement in the literature regarding acetone peroxide: ''Its volatility in an open glass is 96% over 94 hours at room temperature.'' |

Synthesis of Acetone Peroxide Using Acetone Diluted with Unknown Solvent |

|

|

|

|

|

|

|

|

|

|

|
|
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

The next day |

|
|
By the way, have you ever seen what happens when 30% hydrogen peroxide gets on human skin? (See photo below).
*** Кстати, вы когда-нибудь видели, что происходит, когда 30% перекись водорода попадает на кожу человека? (См. фото ниже). |
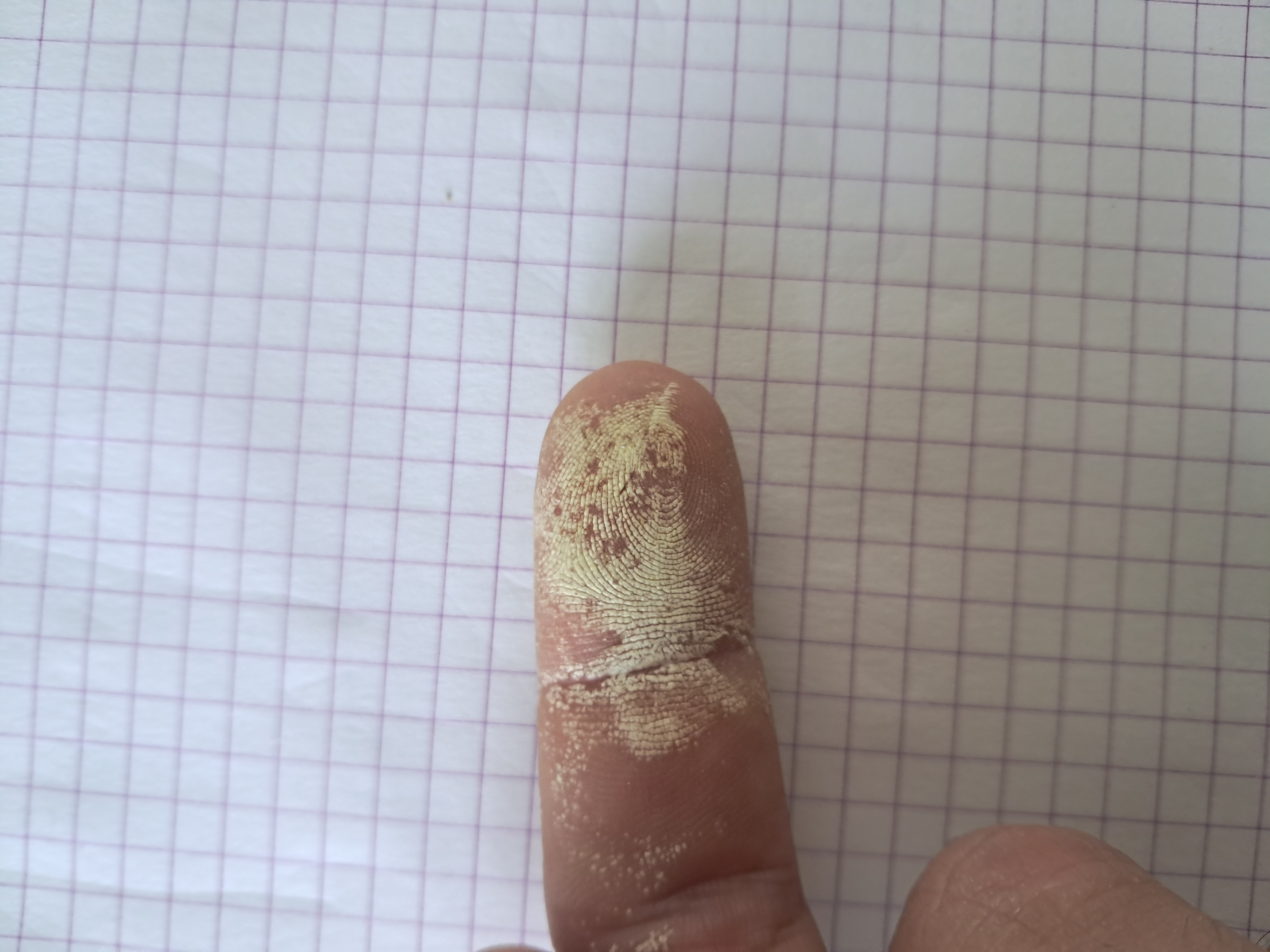
Hydrogen peroxide on the skin |
|
Having noticed a mistake in the text, allocate it and press Ctrl-Enter
Experiments with Fake (Diluted) Acetone - part 5
While waiting for the acetone peroxide to crystallize from the solution, I conducted several experiments using low-quality acetone (diluted with unknown solvents). I poured the organic layer, which had collected in a separatory funnel above the hydrogen peroxide solution (refer to the previous part of this article), into a glass. To this liquid, I added 13.5% hydrochloric acid and stirred. An emulsion formed but quickly separated. Initially, the aqueous layer turned orange, then became discolored - due to some peroxide entering the glass along with the organic solvent.
Эксперименты с поддельным (разбавленным) ацетоном - часть 5 Next, I poured the original "acetone" into another glass, added hydrochloric acid, and stirred again. After the emulsion broke down, the aqueous layer turned a deep orange, while the organic layer remained colorless. Interestingly, the orange color in the aqueous layer persisted for several hours. I covered the glass with a Petri dish and left it overnight. By morning, the organic layer had evaporated, and the orange color remained only in the upper part of the aqueous layer, with the rest of the solution becoming colorless. I could have attempted to identify the substances added by the manufacturer to the acetone. However, I chose not to pursue it, especially given that other batches of this "acetone" might have completely different chemical compositions. |

The organic layer (immiscible with water) |

|

|

|

|

+ HCl |

|

|

|

|

|

|

|

The fake (diluted) acetone |

+ HCl |

|

|

|

|

|

|

|

The next day |

|
|
Having noticed a mistake in the text, allocate it and press Ctrl-Enter
The colleague involved in polymer synthesis did not specify how much acetone peroxide he needed for the polymerization reactions. However, I am certain that the required amount is much greater than the 0.04 g of acetone peroxide I obtained in the last synthesis. I believe that several grams of acetone peroxide are necessary for this purpose. If the polymerization scale is large, then likely several tens of grams of this initiator will be required (which is VERY dangerous). At the time of writing, I do not know the exact amount needed.
In any case, my first step was to develop a method for synthesizing acetone peroxide using the available low-quality raw materials. After that, I aimed to obtain the required amount of peroxide or determine that using diluted acetone for synthesis is impractical. I added 60 ml of a 30% hydrogen peroxide solution to 60 ml of acetone and stirred. Initially, an emulsion formed, which quickly separated. An organic solvent, immiscible with water, collected on top, with a volume of about 40 ml. Below this layer was a solution of hydrogen peroxide and acetone in water. I separated the aqueous phase using a separating funnel and cooled it in an ice bath - this time I did not neglect cooling, recalling how hot the reaction mixture became last time. For the current synthesis, I chose to use concentrated hydrochloric acid rather than diluted acid. However, I had run out of concentrated acid, but a colleague helped me out. I added 30 ml of concentrated hydrochloric acid to the reaction mixture, which contained hydrogen peroxide and acetone, in an ice bath while stirring continuously. At first, the mixture turned orange, but the color quickly faded. The liquid became cloudy, as oxygen bubbles formed, causing the turbidity - once again, no solid phase precipitated. I left the glass containing the reaction mixture in an ice bath. Over time, the release of gas bubbles slowed down, and the solution became transparent and colorless. No solid phase was observed. About an hour later, I removed the glass from the ice bath and placed it on the table to accelerate the crystallization of acetone peroxide. At first, nothing happened, so I went to the other side of the lab and sat down at the computer to work on an article (on a completely unrelated topic). I managed to write about a page when I suddenly noticed a sharp, unpleasant smell. I immediately understood what had happened - a sudden, intense reaction had occurred in the glass. When I went to check, I found that 4/5 of the solution had splashed out onto the table. The remaining liquid in the glass continued to bubble. The solid phase still hadn't formed, but the air was filled with the pungent smell of chloroacetone, causing a strong lachrymatory effect. I put on gloves and wiped up the spilled reaction mixture. During the cleanup, I had to run out into the hallway twice - my eyes were unbearably sore and watering. Strangely, the lachrymatory effect dissipated quickly outside the room. Normally, when something like red pepper powder gets in your eyes, wiping them only worsens the pain and irritation. In this case, the opposite happened: when I left the contaminated area and wiped my eyes with a handkerchief, the irritation vanished. Years ago, I had a similar experience during an unsuccessful acetone peroxide synthesis, where a lachrymator formed but didn't have a strong effect - no significant tearing or eye pain. This time, however, the effect was intense. 
Hydrochloric acid is volatile, so if the acetone peroxide precipitate isn't thoroughly washed on the filter, the remaining acid will evaporate during drying. Sulfuric acid, on the other hand, is non-volatile. If sulfuric acid remains in acetone peroxide, it could increase the instability of the compound, making it even more dangerous to handle. Later, I messaged another colleague about the incident, and he responded: "If you use diluted sulfuric acid instead of hydrochloric acid, this by-product can't form. I studied this during my student years. The downside is that it's harder to wash sulfuric acid off the product. I also found out that hexamethylene triperoxide diamine (HMPD) is more stable than acetone peroxide during storage. Acetone peroxide, in particular, tends to recrystallize spontaneously due to sublimation." Before the synthesis, I had considered using diluted nitric acid or even citric acid as alternatives to hydrochloric acid, but not sulfuric acid. Since two colleagues independently recommended diluted sulfuric acid, I decided to try this option for the next synthesis. |

Synthesis of Acetone Peroxide (Failure) |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

Most of the solution splashed out onto the table. Chloroacetone had formed, causing a strong lachrymatory effect
|

|

|
|
By the way, I found a photo in my archive that a friend had shared with me. It shows the aftermath of an explosion involving 0.1 g of lead azide, a compound with initiation efficiency (initiating ability) close to acetone peroxide. As you can see, the metal parts of a retort stand (a clamp holder and a clamp) were destroyed. Their research group didn't include any chemists or military personnel, so you can imagine how terrified they were - my friend didn't try to hide it!
**** Кстати, в архиве нашел фотографию, которой поделился со мной знакомый. На фото результат взрыва 0.1 г азида свинца, который близок к перекиси ацетона по инициирующей способности ("силе взрыва"). Как видите, металлические детали лабораторного штатива разрушены. В их исследовательской группе не было ни одного химика или военного - можно себе представить, как они испугались (собственно, знакомый этого и не скрывал). |

The consequences of the explosion of 0.1 g of lead azide |
|
Комментарии
К1
Очень давно делал эту перекись ( самое смешное, что для похожей цели - полимеризовать стирол или ММА [метилметакрилат]).
Получилась ли полимеризация - уже не помню, но остаток перекиси, стоя в стеклянной банке, возогнался частично к крышке прекрасно сверкающими крупными кристаллами. Прямо алмазики. Открывать банку не рискнул, говорят, что крупные кристаллы чувствительнее мелких. Обернул газетой и поджёг в безопасном месте. Бабахнуло порядочно. Банку тогда отставил и забыл на несколько лет. Так что в замкнутом объёме возгоняется не очень быстро. На воздухе, очевидно, гораздо быстрее. |