Chemistry and Chemists № 1 2025
Journal of Chemists-Enthusiasts
| Content | Chemistry experiments - video | Physics experiments - video | Home Page - Chemistry and Chemists |
|
Chemistry and Chemists № 1 2025 Journal of Chemists-Enthusiasts |
Acetone, Acetone Peroxide, and Chloroacetone - pt. 7, 8 Bad Chemist |
|
Having noticed a mistake in the text, allocate it and press Ctrl-Enter
Synthesis of Acetone Peroxide Using Sulfuric Acid and Acetone Diluted with Unknown Solvent - pt.7
So, I decided to synthesize acetone peroxide using the method described above, but instead of hydrochloric acid, I used dilute sulfuric acid to avoid the formation of chloroacetone.
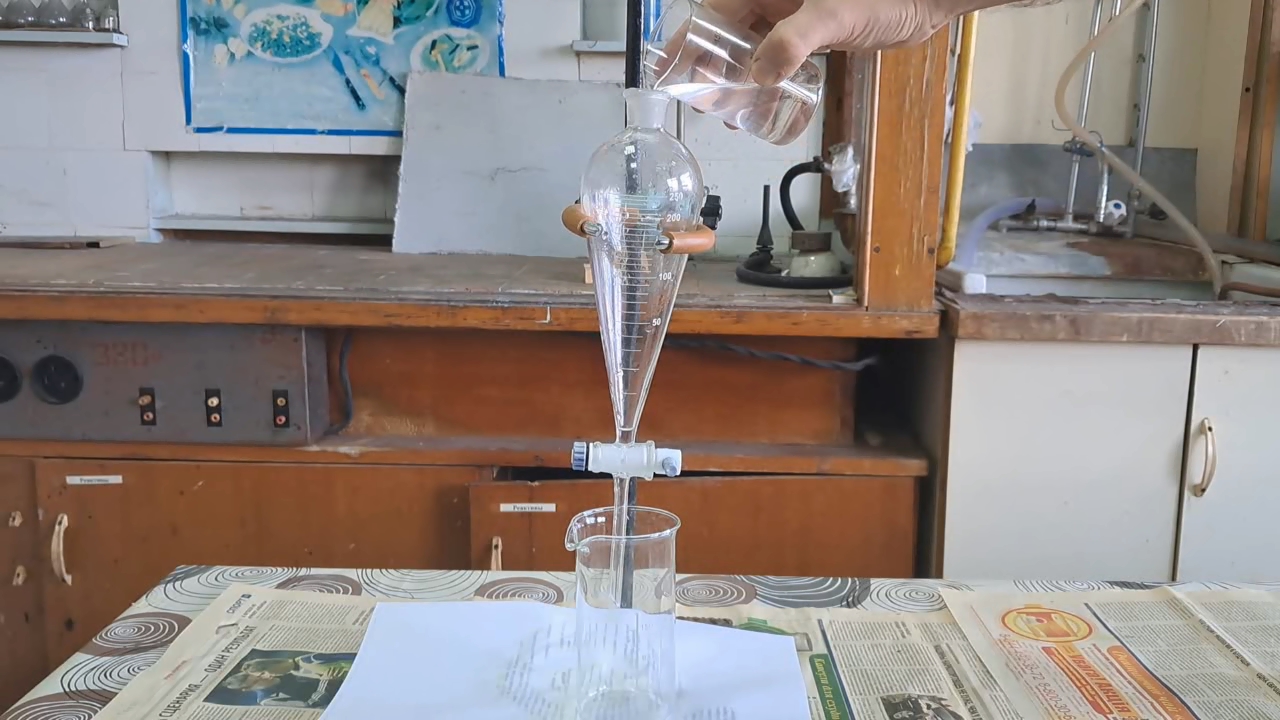
Синтез перекиси ацетона исходя из ацетона, разбавленного неизвестным растворителем и серной кислоты - ч.7 First, I mixed 30 ml of a 30% hydrogen peroxide solution with an equal volume of low-quality acetone. As in the previous case, two immiscible liquid layers formed. I separated the aqueous phase using a separatory funnel and transferred it to a beaker. I then poured the organic phase (the upper layer) into a graduated cylinder to determine its volume. It measured 22 ml. (Let me remind you that this liquid is not used in the synthesis.) Next, I placed the glass with the aqueous solution of acetone and hydrogen peroxide into an ice-water bath. At this point, instead of hydrochloric acid, it was necessary to add dilute sulfuric acid. I had an electrolyte for lead-acid batteries, which is a solution of sulfuric acid. Two bottles of electrolyte were left over from my predecessors. The label indicated a density of "1.27" kg/l, corresponding to a sulfuric acid concentration of 36-37%. I calculated the appropriate amount of sulfuric acid to add, considering that sulfuric acid is dibasic. Based on the calculation, I added 12 ml of the sulfuric acid solution to the reaction mixture and stirred. The liquid immediately became cloudy white - a white precipitate formed. No significant release of oxygen bubbles or noticeable heating of the mixture occurred. Visually, the amount of precipitate was small; it did not settle to the bottom for a long time, remaining as a white suspension. By the way, there were some surprises. When I added sulfuric acid to the reaction mixture, no orange substance formed - the solution remained colorless (or rather, it turned white). Let me remind you that when I added hydrochloric acid to the low-quality acetone used for this synthesis, a bright orange color appeared. I left the reaction mixture in a cold water bath overnight, covering the glass with a Petri dish. Remembering my previous unfortunate experience, I placed everything in a large basin - just in case a violent reaction occurred and the solution splashed out of the glass. My fears were unfounded: by the next day, white flakes were floating in the glass. No liquid had been ejected from the glass, and there were no signs that a violent reaction had occurred during the night. I filtered the precipitate through filter paper, washed it thoroughly with distilled water, and then rinsed it with a small amount of alcohol (to speed up drying). I left the precipitate to dry on filter paper at room temperature. This time, I remembered that acetone peroxide is highly volatile. As soon as the precipitate dried enough to be separated from the paper, I immediately transferred it to dry filter paper and then placed the acetone peroxide into a closed plastic bottle. I weighed the resulting product - it turned out that I synthesized only 0.18 g of acetone peroxide! What can you do with such a small amount unless you plan to detonate it? |

Synthesis of Acetone Peroxide Using Sulfuric Acid and Acetone Diluted with Unknown Solvent |

|

|

|

|

|

|
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

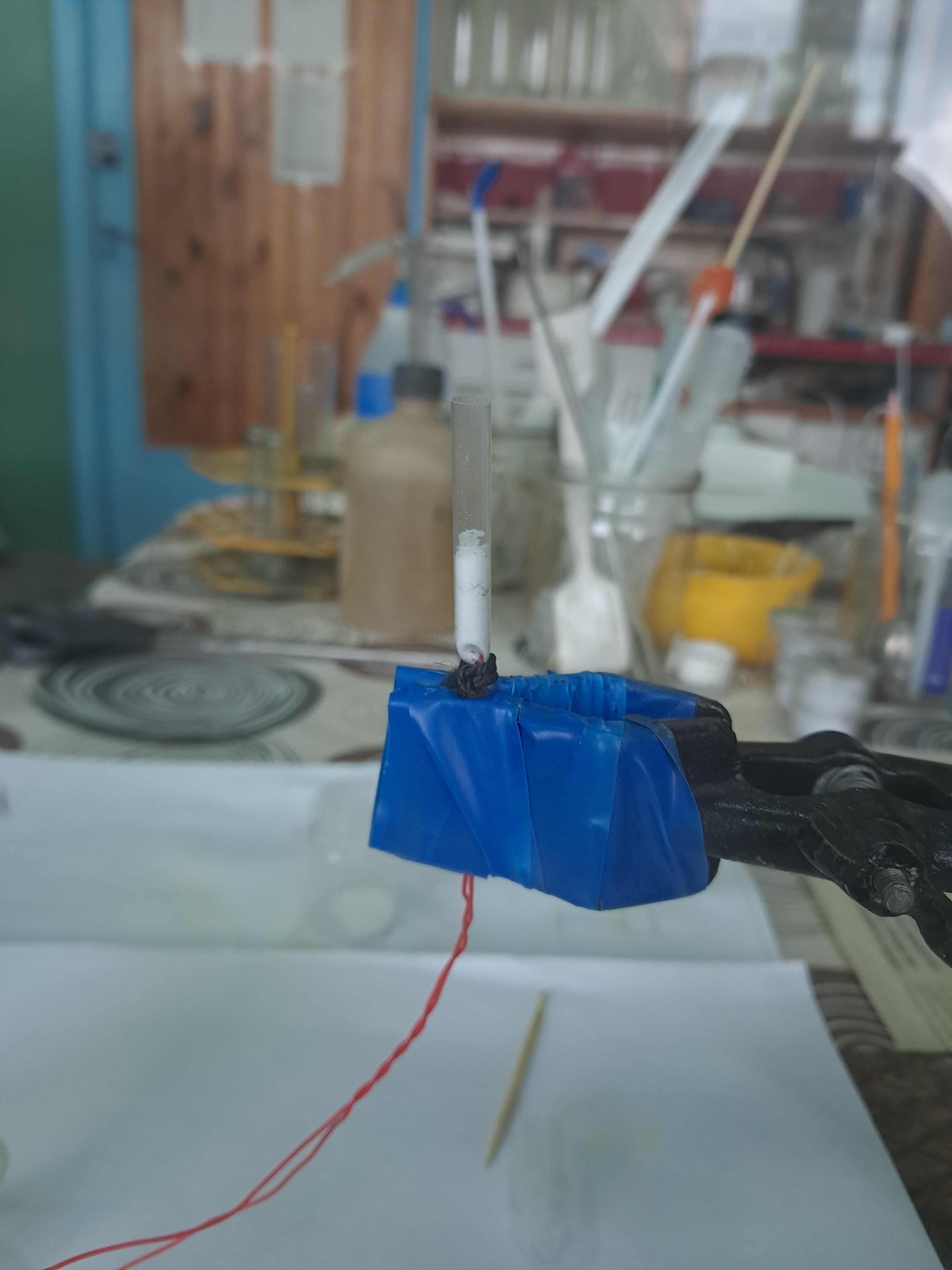
Explosion of Acetone Peroxide (0.05 g) |

|

|

|

|

|
|

|

|

|

|

|

|
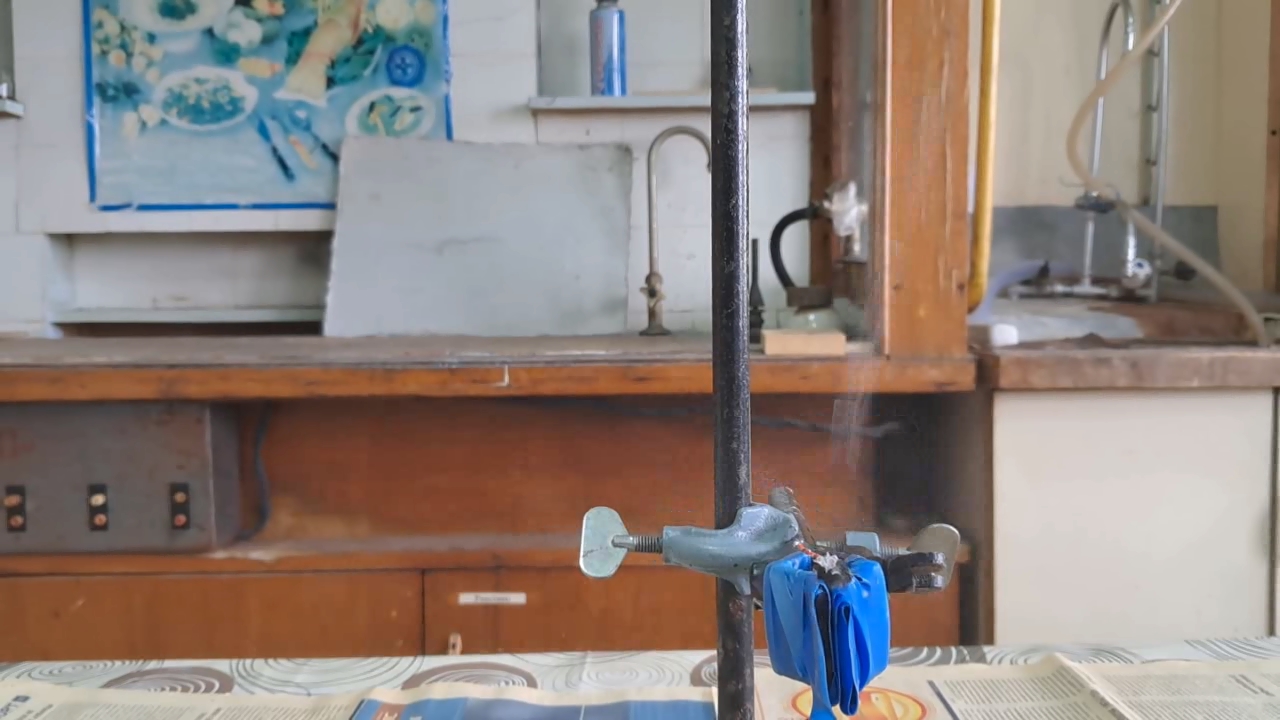
|
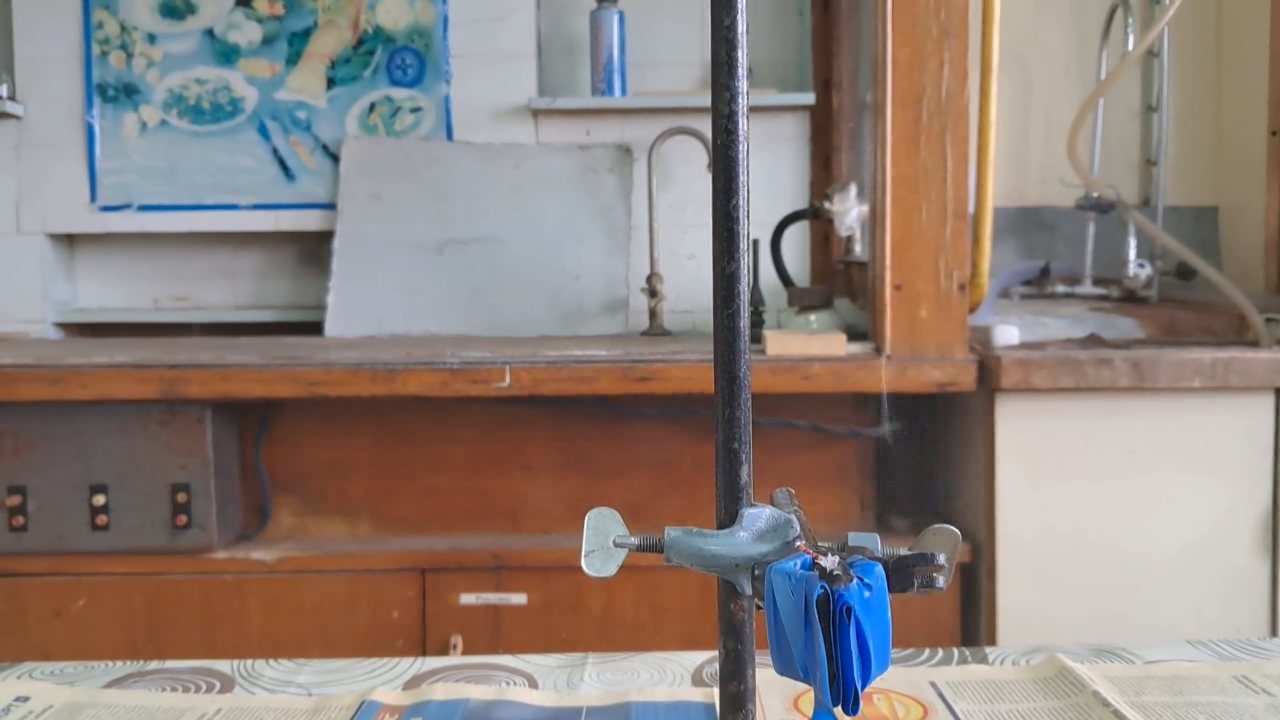
|

|

|

|

|

|

|

The cotton wool |
|
Комментарии
К1
Для получения компактной перекиси ацетона её можно переплавлять на водяной бане. Температура плавления близка к ста градусам. Процесс опасный, лучше проводить дистанционно.
Много лет назад, в студенчестве, мне попался полиэтиленовый контейнер с полусферическими углублениями примерно диаметром по 2 см., не помню от чего. Решил в нём сформовать "таблетки" ГМТД. Насыпал порошок в углубления, слегка утрамбовал, добавил немного стирола и поставил на полку. Идея была в полимеризации стирола. В одном из экспериментов через несколько часов самопроизвольно произошёл взрыв. Пострадали динамики звуковой колонки. |