Chemistry and Chemists № 1 2025
Journal of Chemists-Enthusiasts
| Content | Chemistry experiments - video | Physics experiments - video | Home Page - Chemistry and Chemists |
|
Chemistry and Chemists № 1 2025 Journal of Chemists-Enthusiasts |
Acetone, Acetone Peroxide, and Chloroacetone - pt. 11, 12 Bad Chemist |
|
Having noticed a mistake in the text, allocate it and press Ctrl-Enter
The benzoyl peroxide molecule, (BzO)2, consists of two benzoyl radicals (C6H5-C(=O)-, Bz) linked by a peroxide bridge (-O-O-). This oxygen-oxygen bond is relatively weak, which allows the molecule to easily dissociate and form two free radicals in a homolysis reaction.
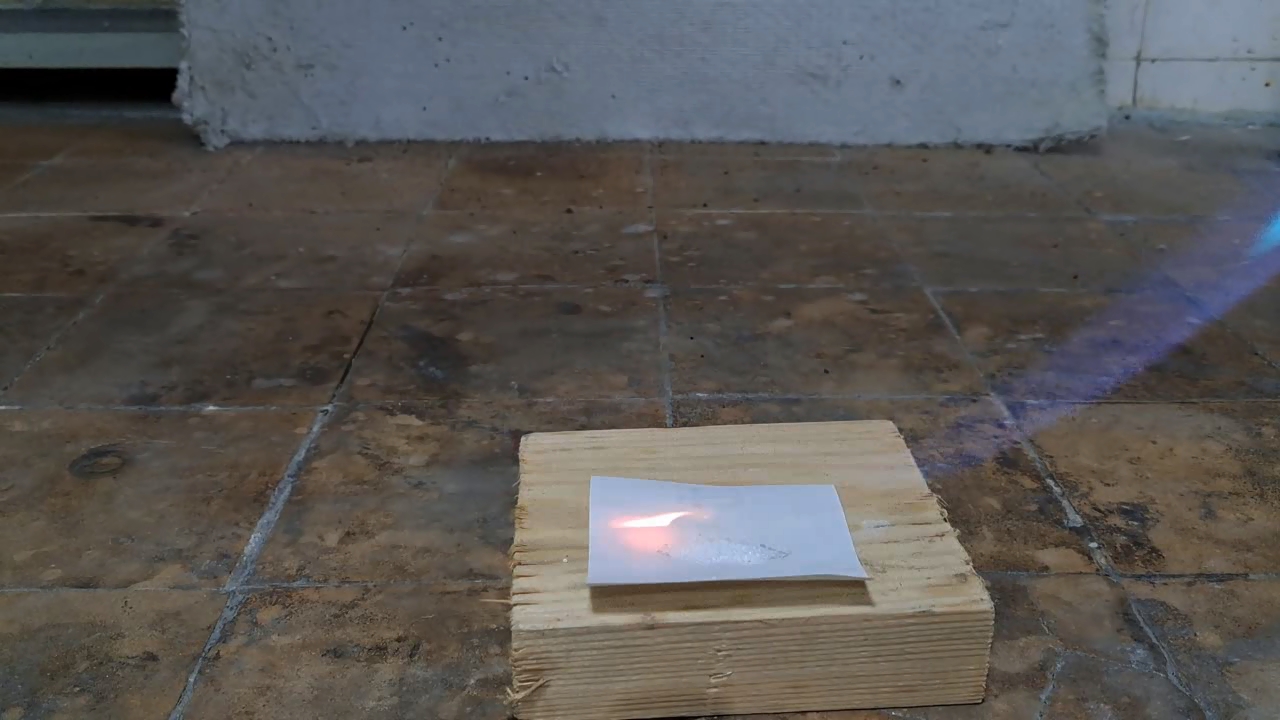
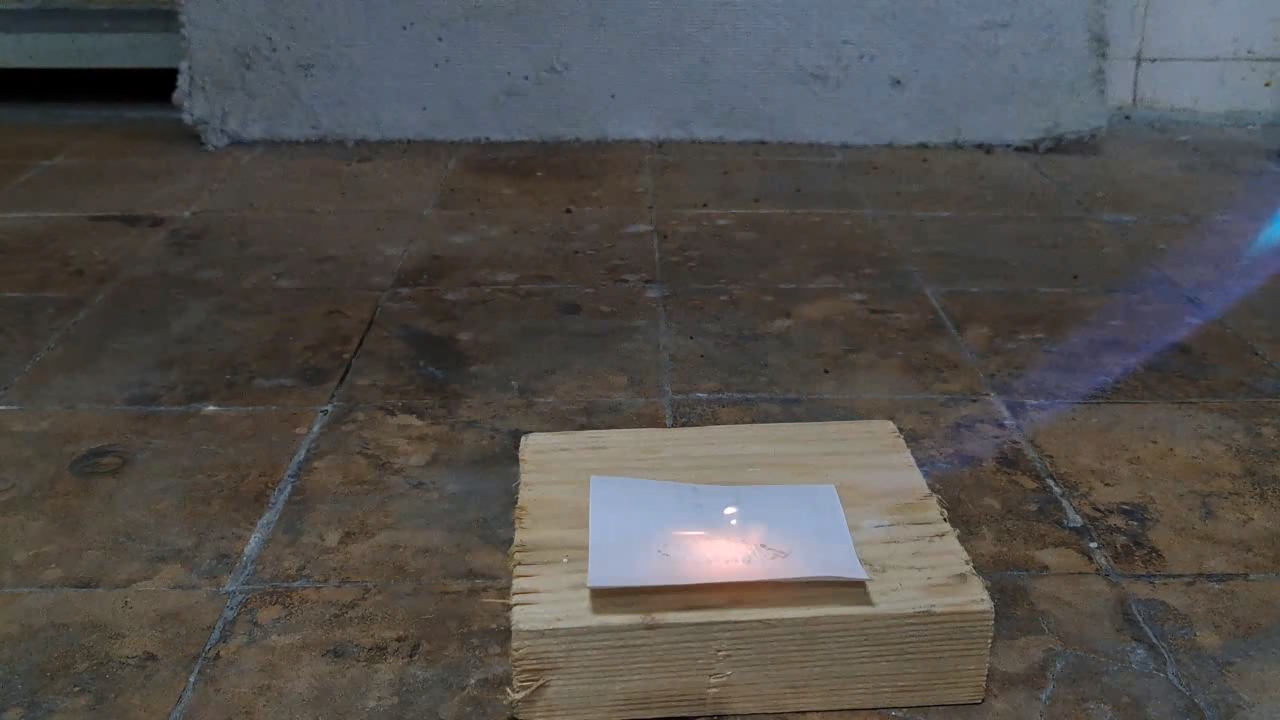
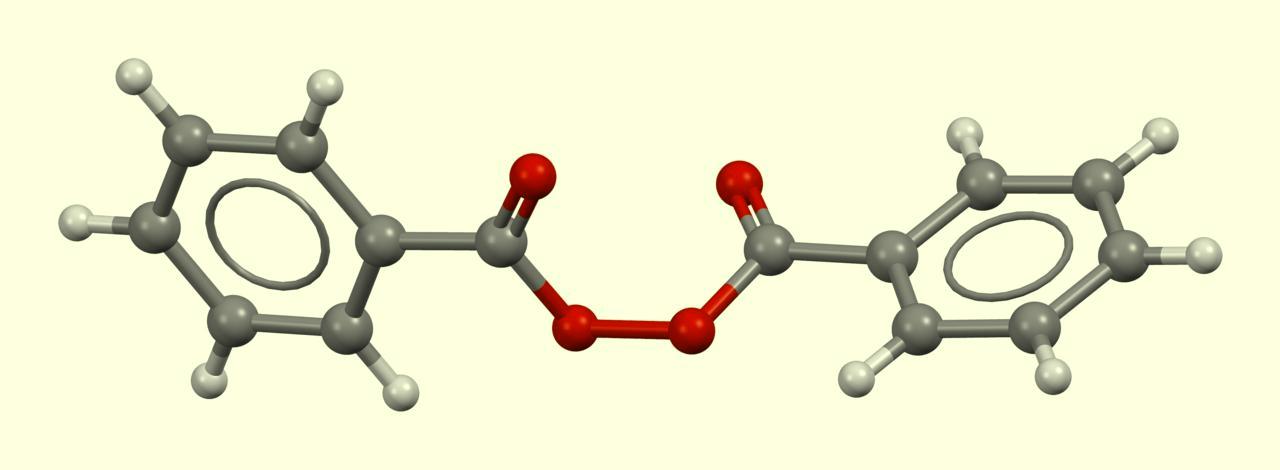 I've read from various authors that benzoyl peroxide can burn intensely when ignited and may even explode due to friction or if a large quantity ignites. However, I want to emphasize again that it is far less hazardous than acetone peroxide. Benzoyl peroxide has other roles too: it's used as a vulcanizing agent, a hardener for epoxy resins, and, due to its oxidizing properties, as a bleaching agent for flour (a food additive) and for tooth whitening (in medical applications). In combination with other pharmaceuticals, it's also a common treatment for acne (an inflammatory skin disease). Benzoyl peroxide is synthesized by reacting benzoyl chloride with hydrogen peroxide in an alkaline solution, a method distinct from those used for other peroxides such as acetone peroxide, methyl ethyl ketone peroxide, and hexamethylene triperoxide diamine (HMTD). To confirm that a sample is indeed benzoyl peroxide, the most rigorous approach is iodometric titration in an acetone solution, which allows you to determine the benzoyl peroxide content precisely. Although I work in an analytical chemistry department, I decided against a quantitative analysis. Instead, I could have done a simpler qualitative test with potassium iodide in an acidic solution; if iodine is released, this indicates the presence of benzoyl peroxide (or another oxidizer). I had potassium iodide on hand (along with cadmium, barium, and sodium iodides), but I decided to try a flame test instead. 
Cadmium, barium and potassium iodides 





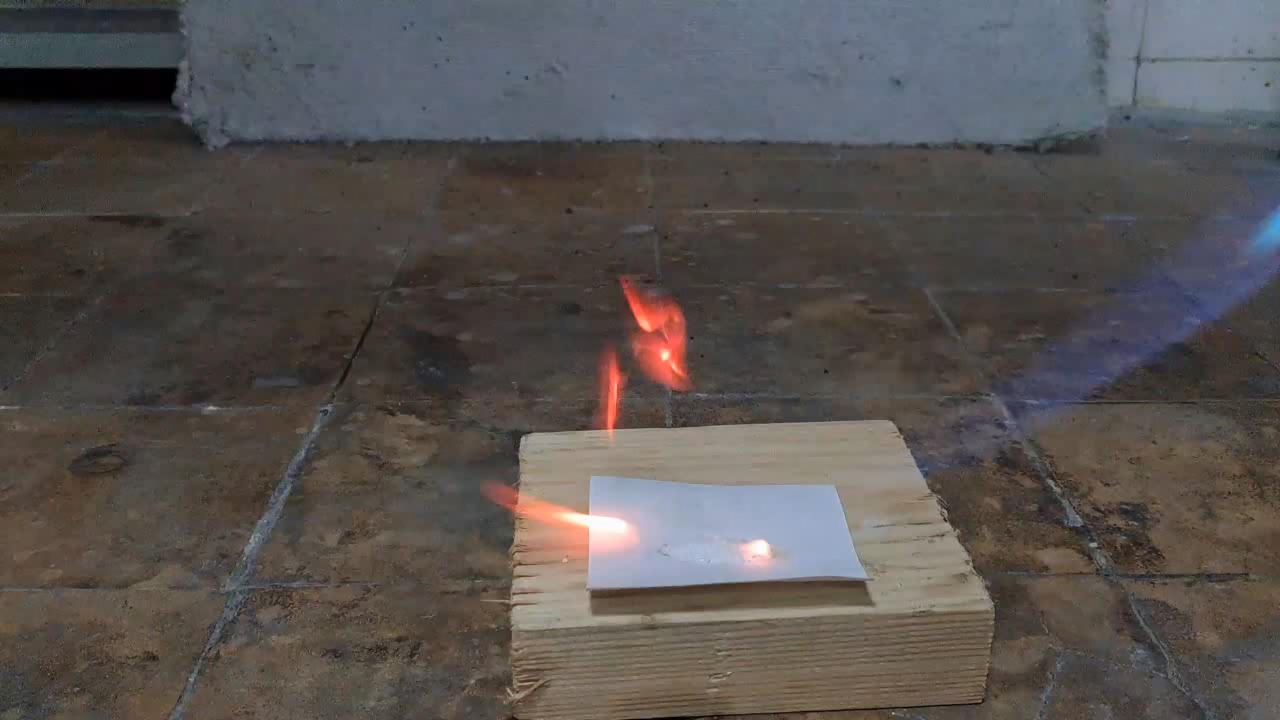
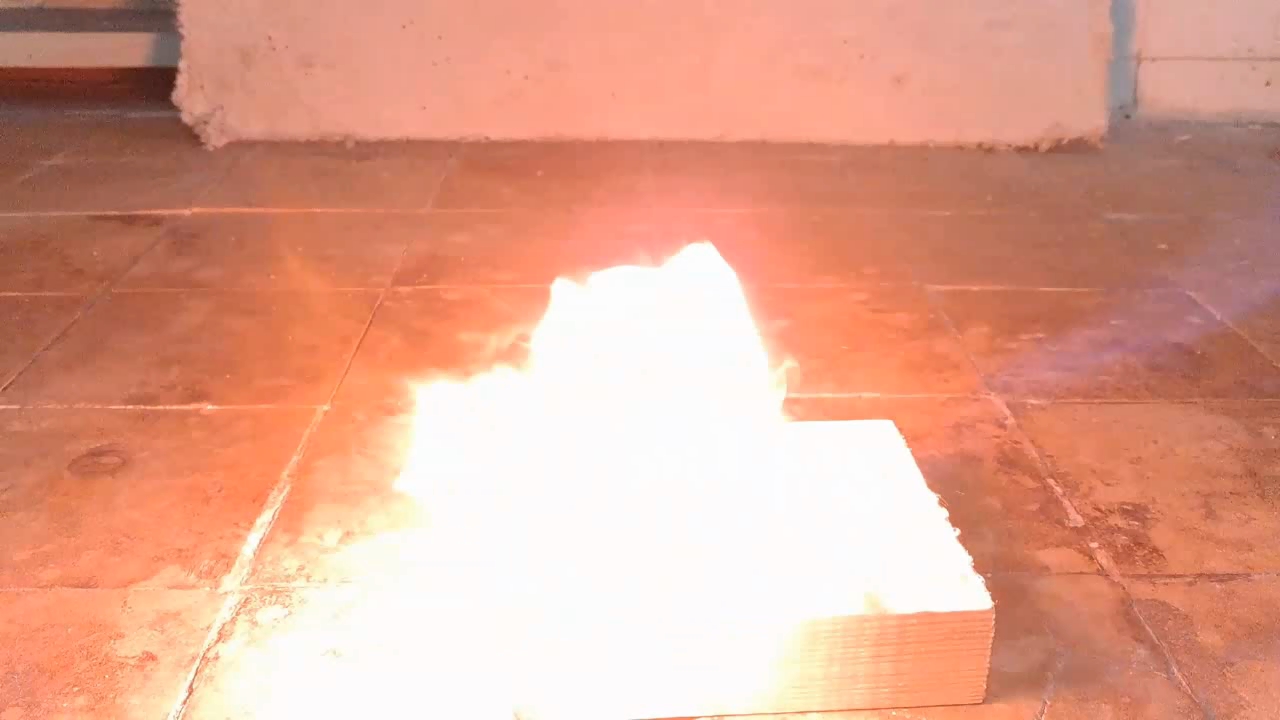
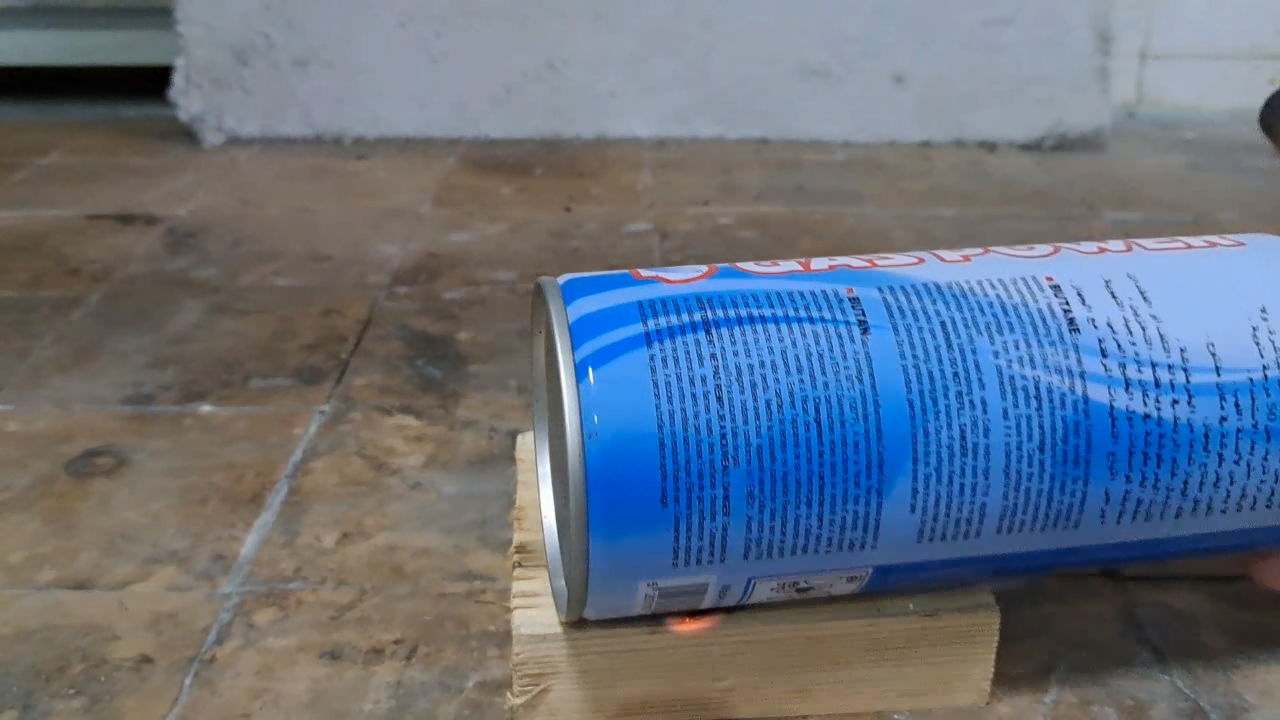
For a second test, I placed some benzoyl peroxide on aluminum foil (since foil doesn't burn) and aimed the torch at it. A series of yellow flashes followed, with significant soot formation. After the combustion ceased, a yellowish residue remained on the foil, similar to an organic resin. For comparison, acetone peroxide or HMTD burns completely when ignited, leaving no solid residue. In conclusion, this sample is benzoyl peroxide, although it may contain decomposition products or other impurities. I expect these won't interfere with its use in the polymerization reaction. |

Benzoyl Peroxide and Fire |

|

|

|

|

|

|

|

|
|

|
|

|

|

|
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|

|

|

|

|

|

|

|
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
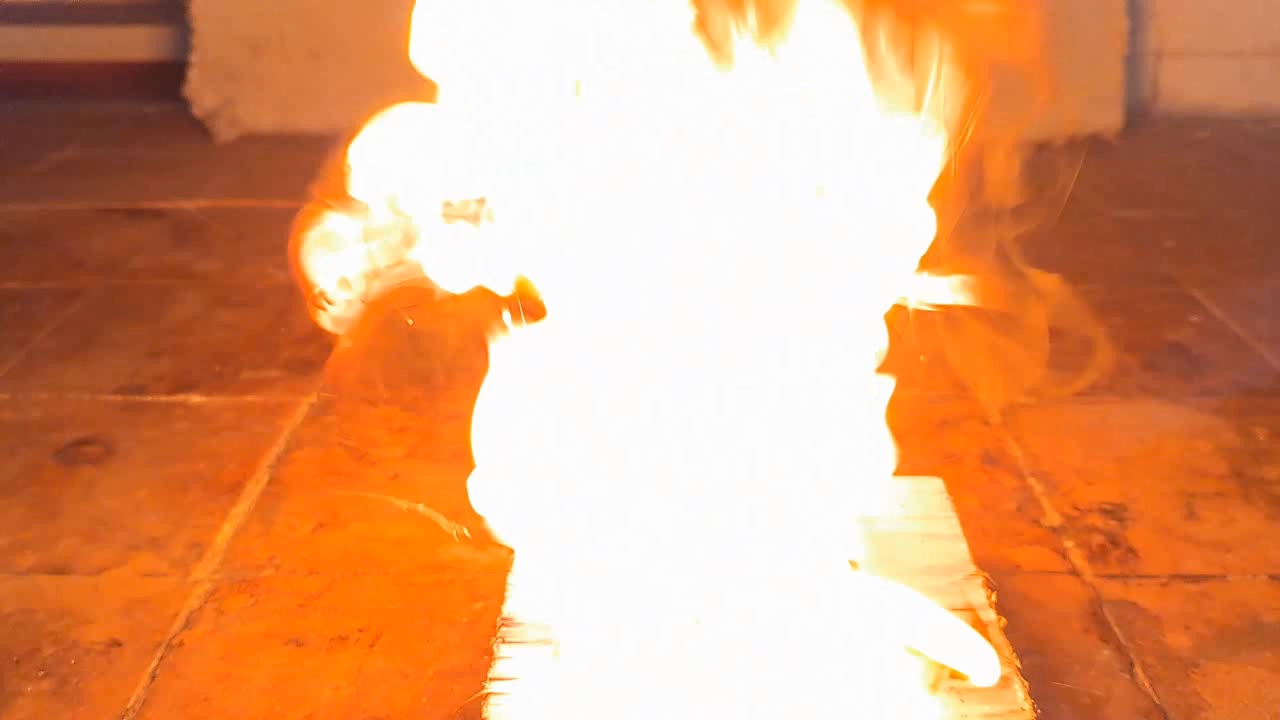
|
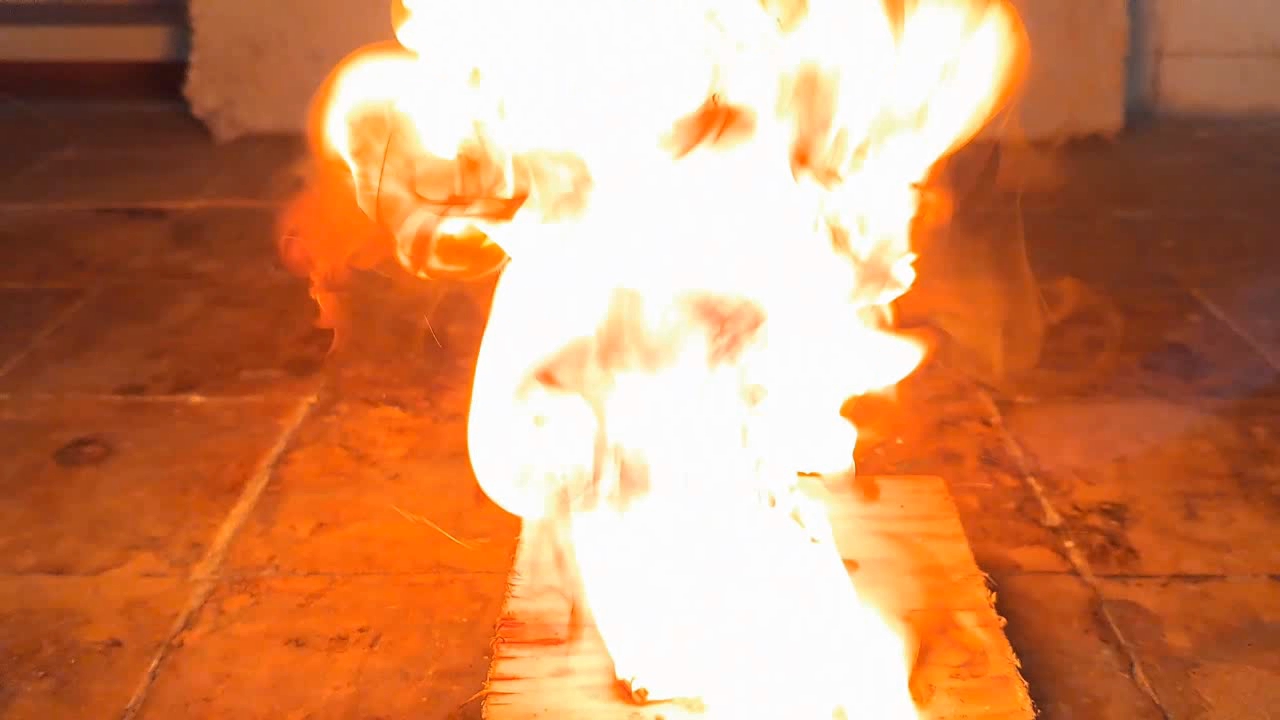
|

|

|

|

|
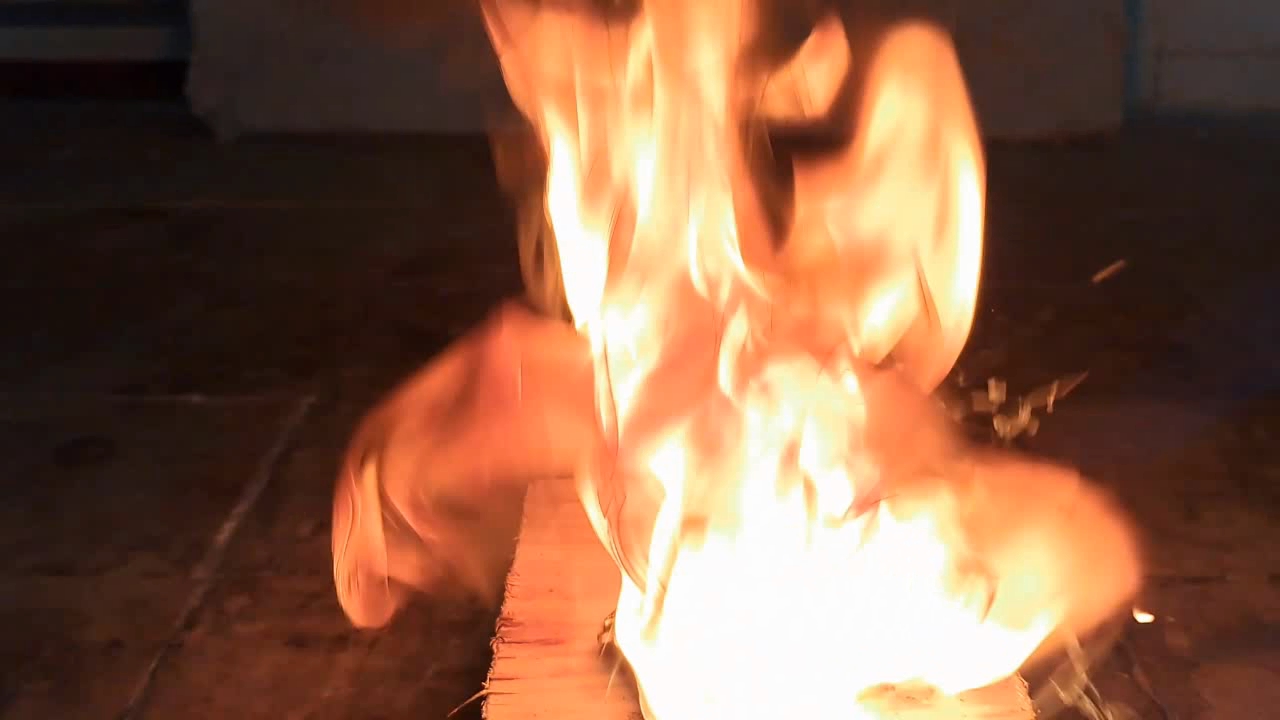
|

|

|

|

|

|
|
Having noticed a mistake in the text, allocate it and press Ctrl-Enter
Synthesis of Acetone Peroxide Using Good Quality Acetone - Part 12
The need to synthesize acetone peroxide disappeared, as this substance had been replaced with less hazardous benzoyl peroxide. Nevertheless, the desire to achieve its synthesis persisted. Why? My past failures were disappointing, and I wanted to succeed - despite the fact that the synthesis had no practical value anymore. Additionally, I now had a little high-quality acetone.
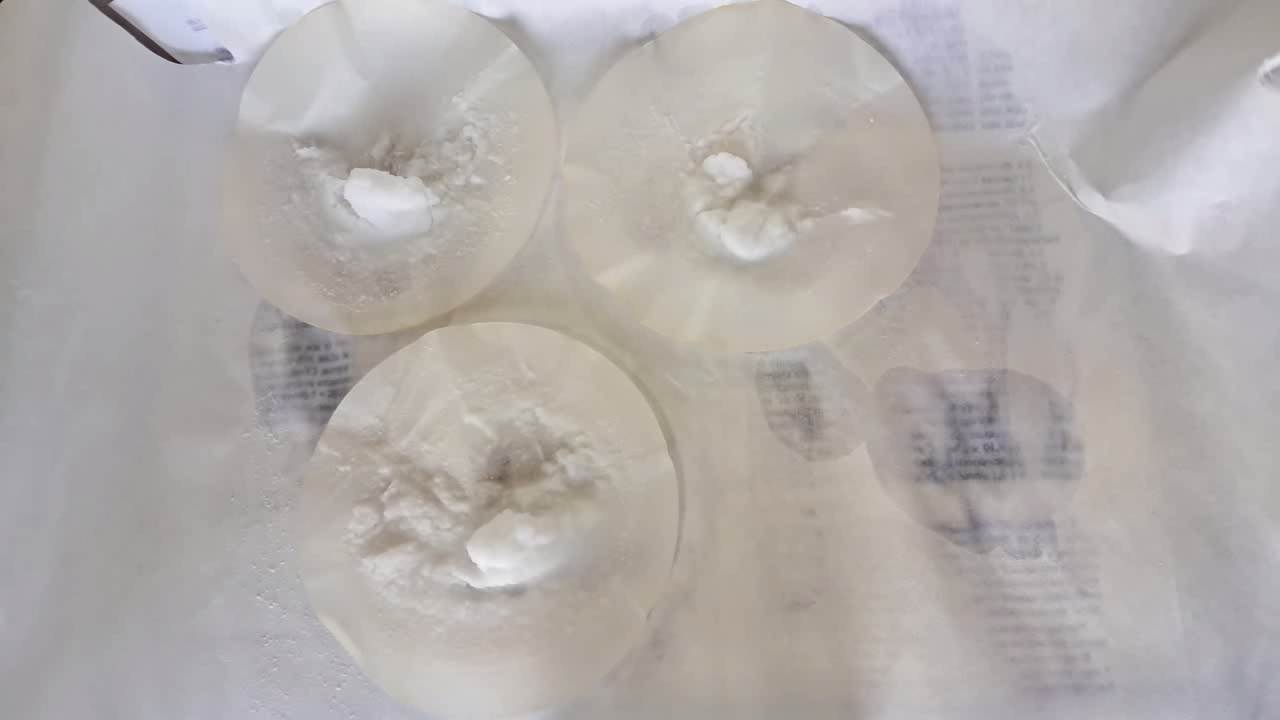
Синтез перекиси ацетона с использованием ацетона хорошего качества - часть 12 I hesitated for some time before beginning. Perhaps the deciding factor was the temperature in the laboratory. Summer had ended, and with it, the heat. Winter was approaching, and the laboratory was cold. As you already know, the synthesis of acetone peroxide requires cooling. I prepared the required reagents: a 30% hydrogen peroxide solution, acetone, and dilute sulfuric acid (lead car battery electrolyte). I poured 30 ml of acetone into a beaker, added 30 ml of hydrogen peroxide solution, and mixed them thoroughly. The acetone I used in my previous experiments contained hydrocarbons, and I feared the same issue might occur with this new batch of acetone. Fortunately, my concerns were unfounded. The liquids mixed completely, forming a clear, colorless solution without any separate hydrocarbon phase. Next, I added 10 ml of dilute sulfuric acid to the beaker and stirred the mixture. Initially, the gas bubbles formed, which made the mixture cloudy. However, these bubbles soon rose to the surface, and the solution became clear and colorless again. At this stage, no solid phase had formed. While filming the experiment, I realized I had forgotten a critical step. The reaction mixture should have been cooled in an ice bath before adding the acid! A colleague touched the beaker and remarked that it had become hot. I checked this and found it was indeed warm, though not hot. Of course, I placed the beaker in an ice bath to cool the reaction mixture. I was momentarily distracted, saying goodbye to my colleague. During this time, the solution turned white and cloudy, indicating the formation of the precipitate. So, I called him back to observe: - Come here and see for yourself! Our candidate may have lost the election, but our synthesis will succeed! - I feel like the synthesis has already succeeded. - Not yet. There must be a massive precipitate at the bottom for that. The amount of precipitate gradually increased. After an hour, a substantial amount had accumulated. I considered filtering it immediately but decided against it. It was the end of the day, and I did not want to leave acetone peroxide to dry unattended. This decision proved to be a good one. By morning, the precipitate had significantly increased in volume, occupying most of the solution. Despite the cold, the reaction mixture emitted a strong odor of acetone, which irritated the eyes when the beaker was brought close. In hindsight, I should have added more hydrogen peroxide yesterday to increase the yield, as some of the acetone had not reacted. I filtered the precipitate using small ashless filters and washed it with distilled water, then with alcohol. To expedite the process, I set up three funnels in parallel. As in previous experiments, the product retained a significant amount of liquid, which required carefully squeezing the filters containing the precipitate to remove it. This was risky, as wet paper tears easily. To dry the acetone peroxide, I placed the filters inside a plastic tray lined with sheets of dry filter paper to absorb liquid. The drying process was slow due to the low laboratory temperature (8°C). Some crystals formed into relatively large, shiny plates that shimmered under the lamp's light. They were visually striking, but I reminded myself of the risks: large crystals of organic peroxides are particularly dangerous. Once the acetone peroxide began separating from the wet paper, I transferred it to a PET bottle and sealed it. The product, still damp, formed viscous lumps and adhered to the funnel, stirring sticks, and other surfaces. Naturally, the substance required further drying. Why did I halt the drying process and store it in a bottle? The reason was purely psychological: I wanted to prevent product loss due to evaporation, a problem I had encountered in earlier experiments. Further drying could be performed later if necessary. The wet precipitate weighed 13.8 g. |

Synthesis of Acetone Peroxide (Using Good Quality Acetone) |

|

|

|

|
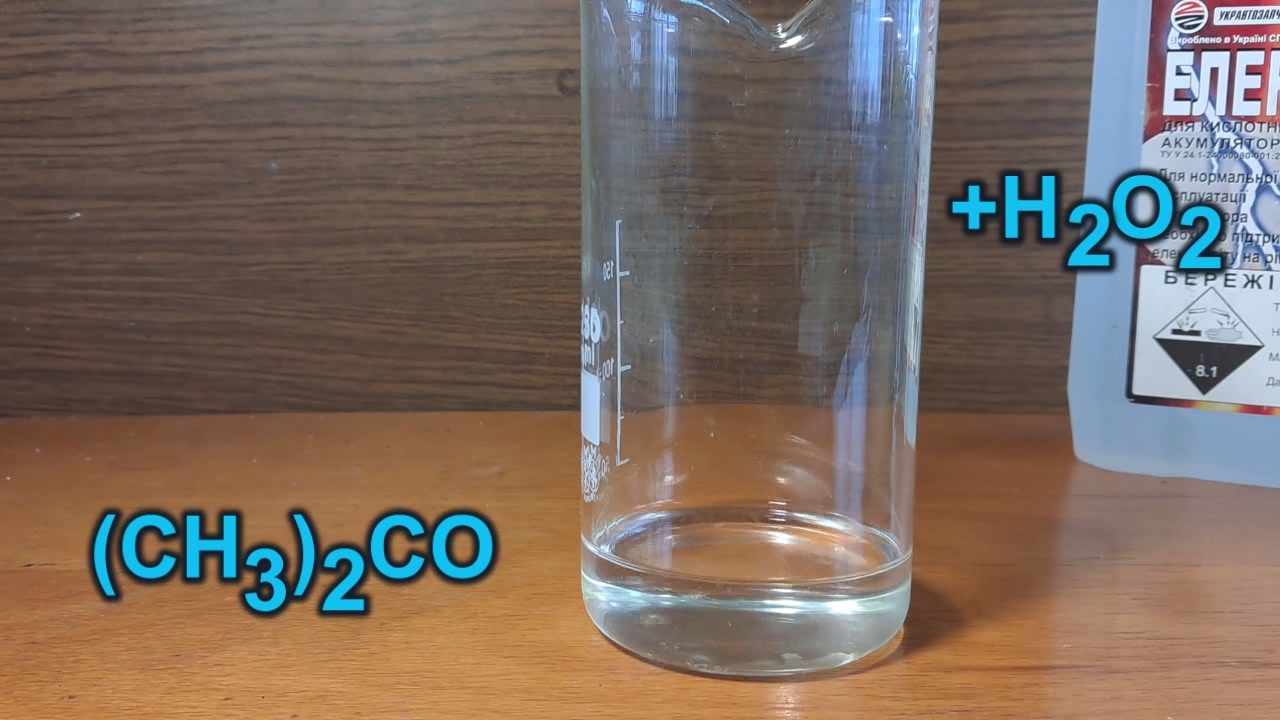
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|