Chemistry and Chemists № 1 2026
Journal of Chemists-Enthusiasts
| Content | Chemistry experiments - video | Physics experiments - video | Home Page - Chemistry and Chemists |
|
Chemistry and Chemists № 1 2026 Journal of Chemists-Enthusiasts |
Electrospinning - pt.20, 21 Chemist |
|
Having noticed a mistake in the text, allocate it and press Ctrl-Enter
Dissolving Expanded Polystyrene in Ethyl Acetate - Part 20
My first attempt to electrospin polymethyl methacrylate was unsuccessful. The New Year was just a few days away, and I wanted to achieve a positive result before the end of the year. So I returned to polystyrene and ethyl acetate. This solvent allowed me to obtain electrospun polystyrene, but I encountered a problem with incomplete evaporation of the ethyl acetate during the process. Splashes of the solution landed on the collector, gluing and dissolving the already formed fiber layer. To solve this problem, I planned to reduce the solution flow rate and use a heater. First, the polystyrene foam needed to be dissolved.
Растворение вспененного полистирола в этилацетате - Часть 20 I weighed 1.830 g of expanded polystyrene and 10.050 g of ethyl acetate. I used large, long pieces of expanded polystyrene-they were easier to dissolve, especially if I needed to simultaneously film the process. I dipped the piece of polystyrene into the solvent. The material quickly "melted" upon contact with the liquid, releasing gas. I continued the process. I gradually dissolved all the polystyrene, creating a cloudy solution. By the way, when I broke up pieces of expanded polystyrene with my hands, the polymer became highly electrified, causing the pieces to stick to my gloves and refuse to fall into the solvent. The gloves were dry at the time. When I tried to place the crushed pieces into the bucket containing the expanded polystyrene, some of the pieces flew out of the bucket due to electrostatic repulsion. Despite their relatively large size, the pieces were lightweight. A similar effect was observed every time I prepared the expanded polystyrene solution. Electrospun polystyrene also becomes highly electrified, sticking to various surfaces, making it difficult to work with. Unfortunately, I haven't yet captured this phenomenon on video, as it's difficult to multitask. |

Dissolving Expanded Polystyrene in Ethyl Acetate |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
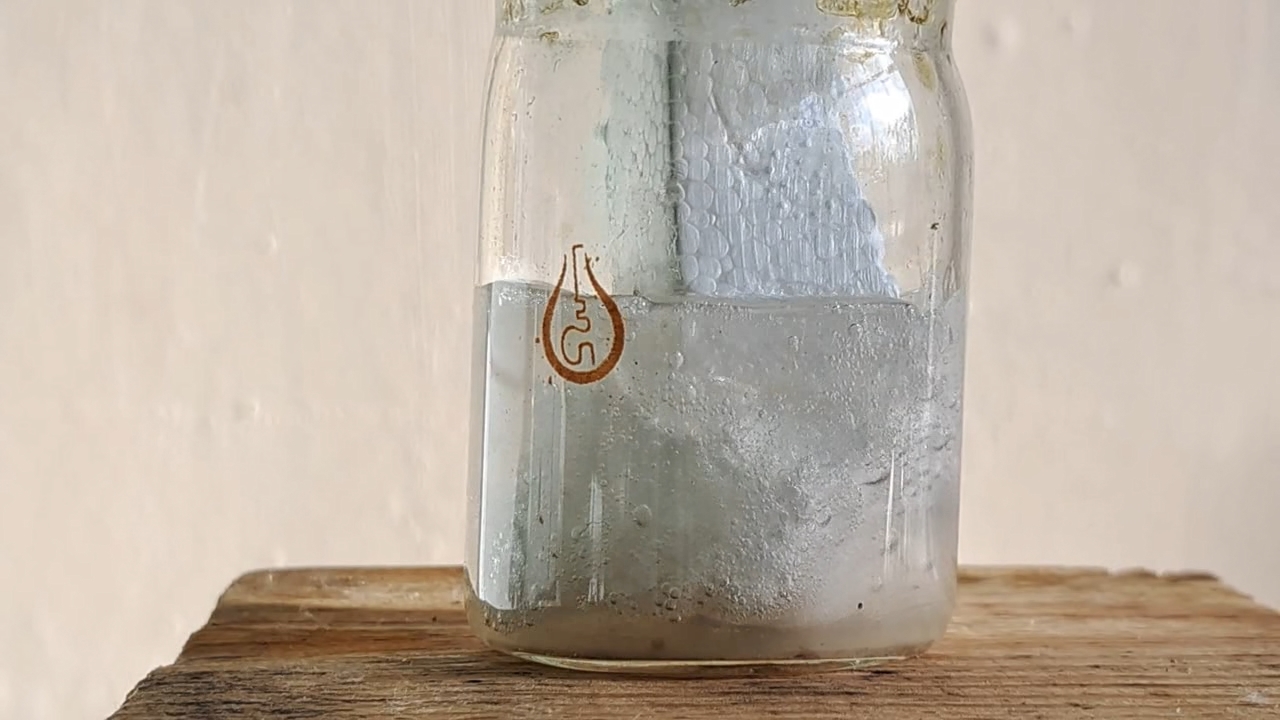
|
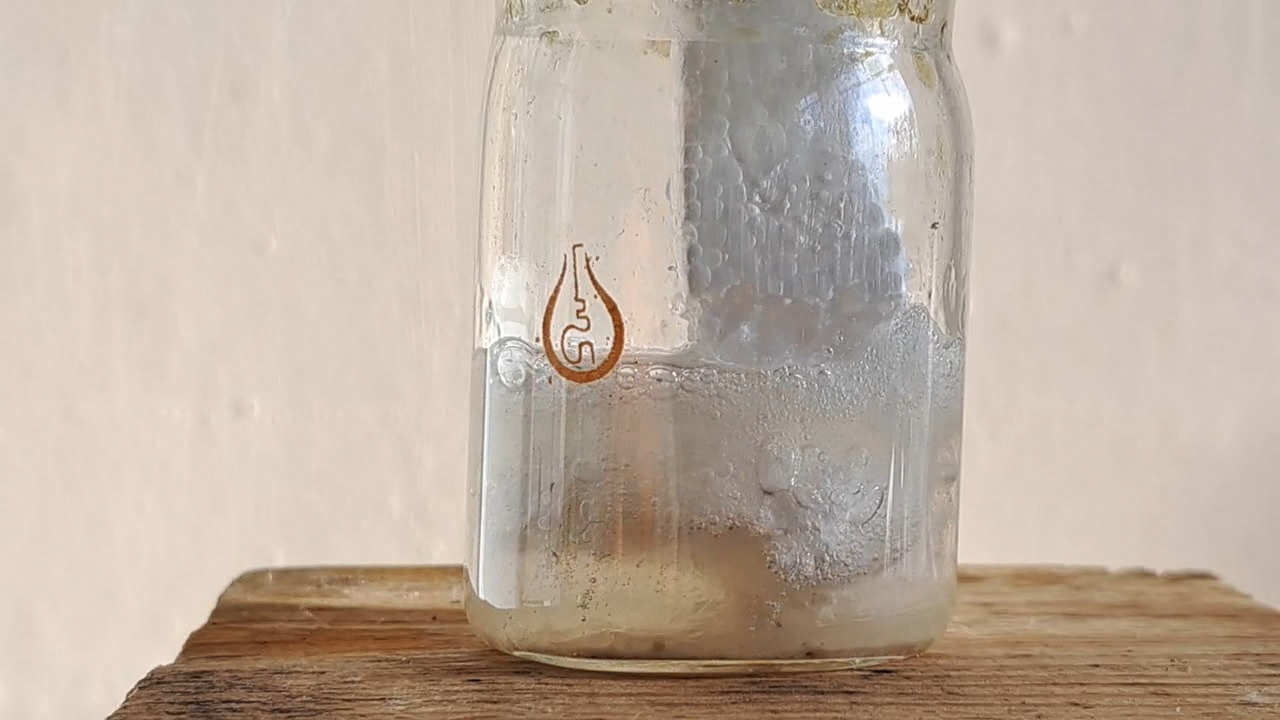
|
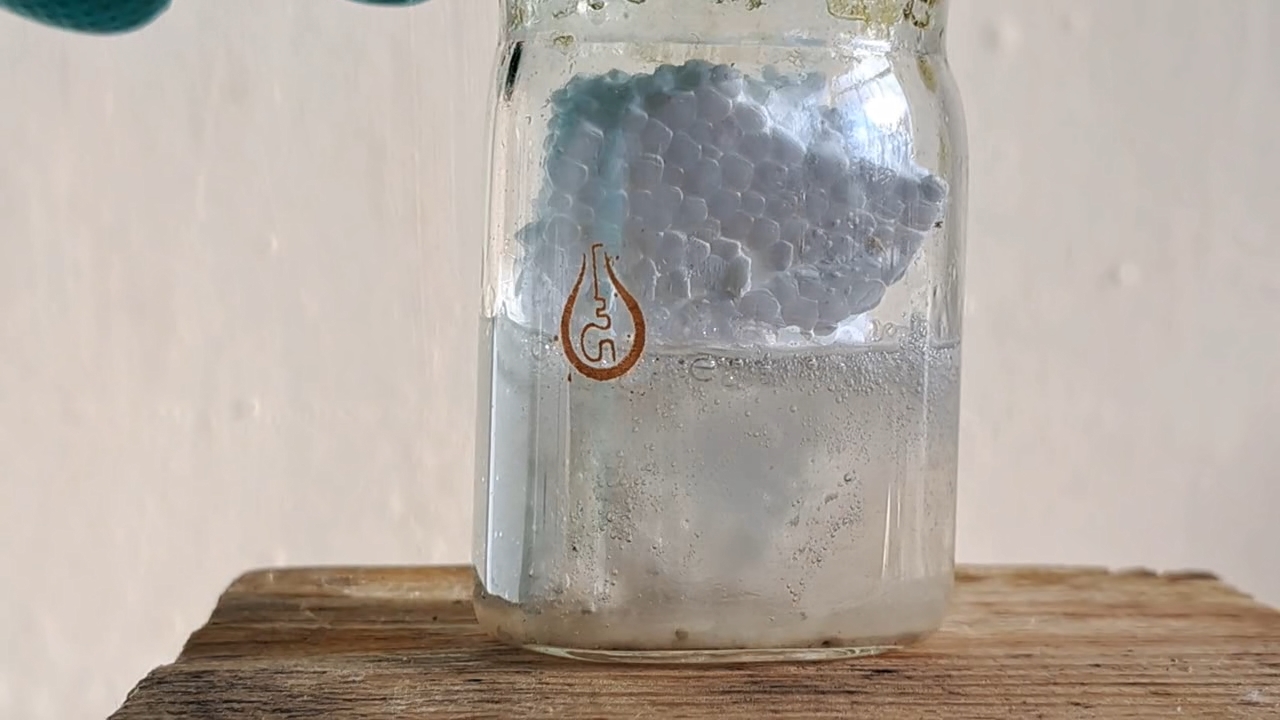
|

|

|

|

|

|
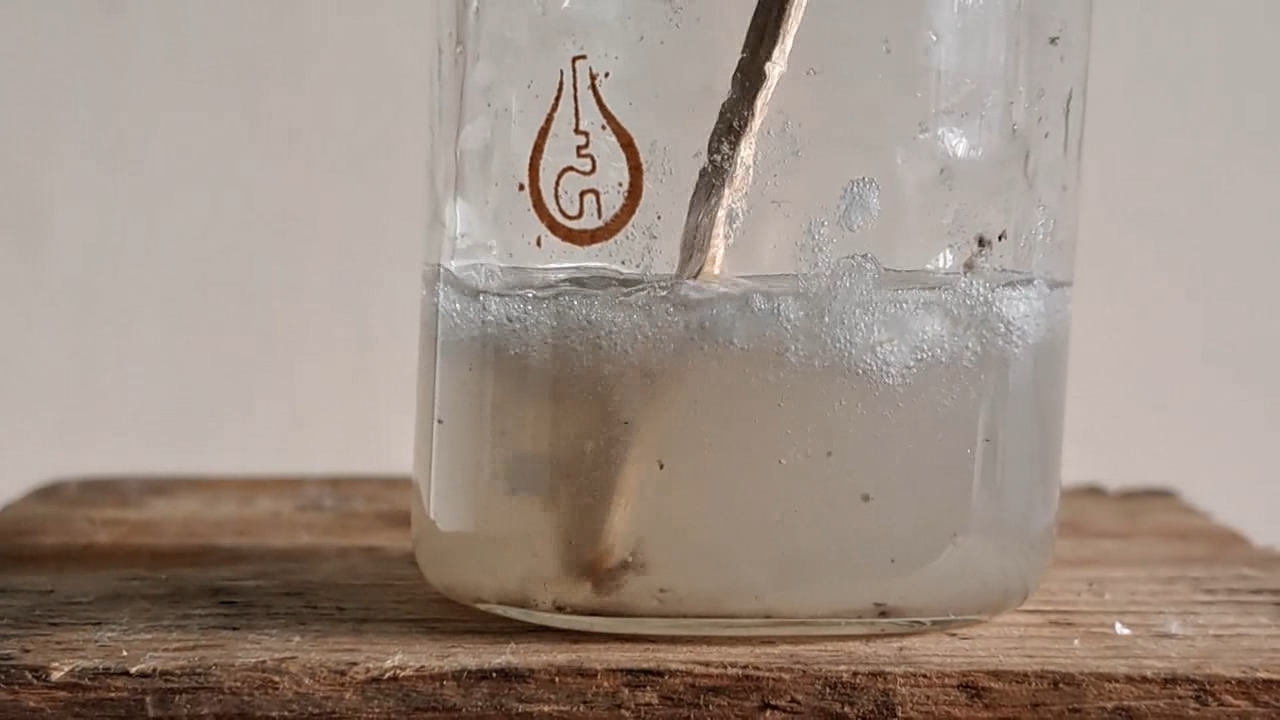
|

|

|
|
Having noticed a mistake in the text, allocate it and press Ctrl-Enter
Electrospinning: Solution of Polystyrene in Ethyl Acetate - Part 21
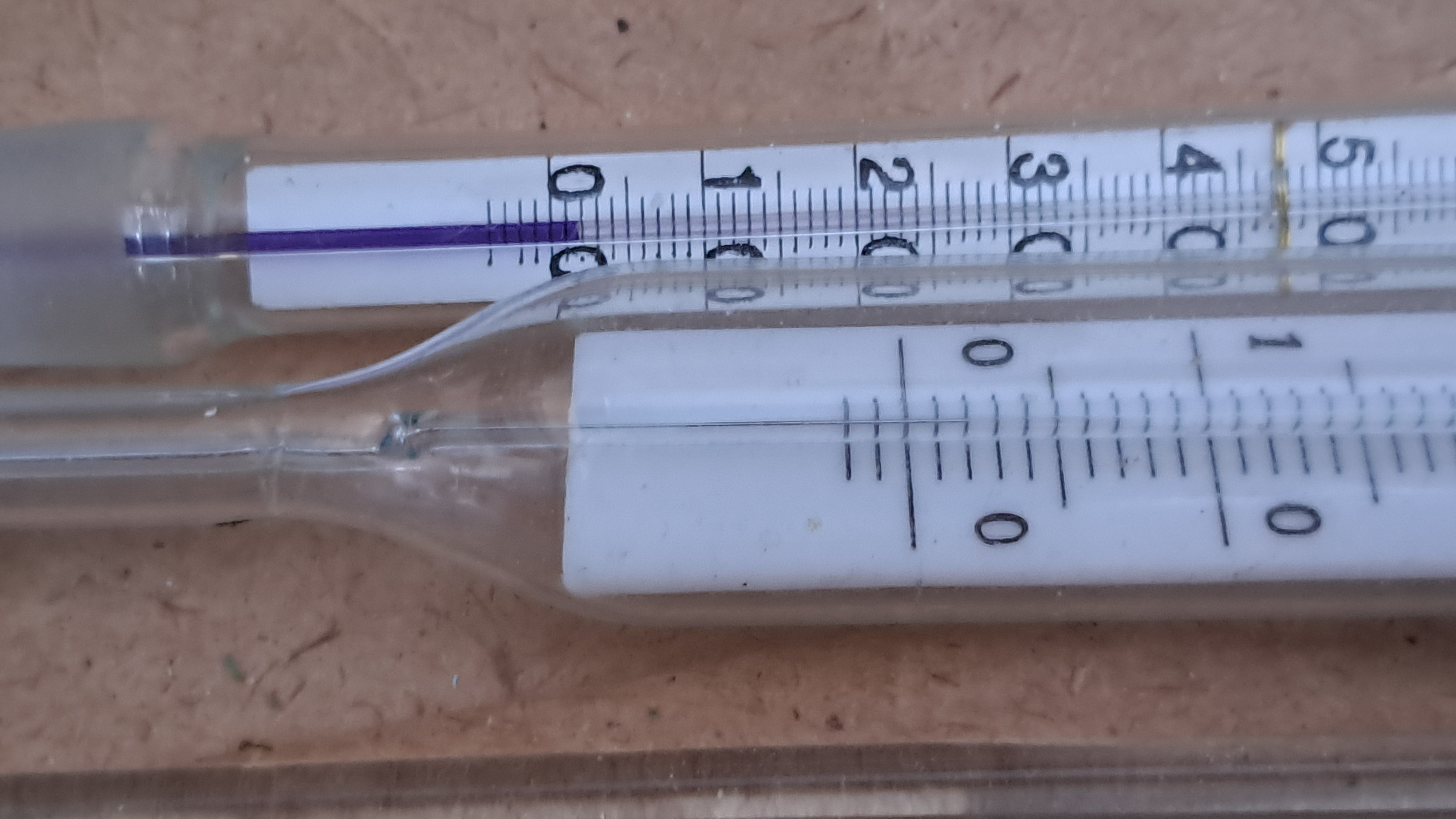
It was sunny and unusually frosty outside. The sun's rays brightly illuminated the laboratory. The room was heated, so in winter the temperature there was usually around 12°C. However, on this day, thanks to direct sunlight, the temperature in the lab reached a whopping 20°C.
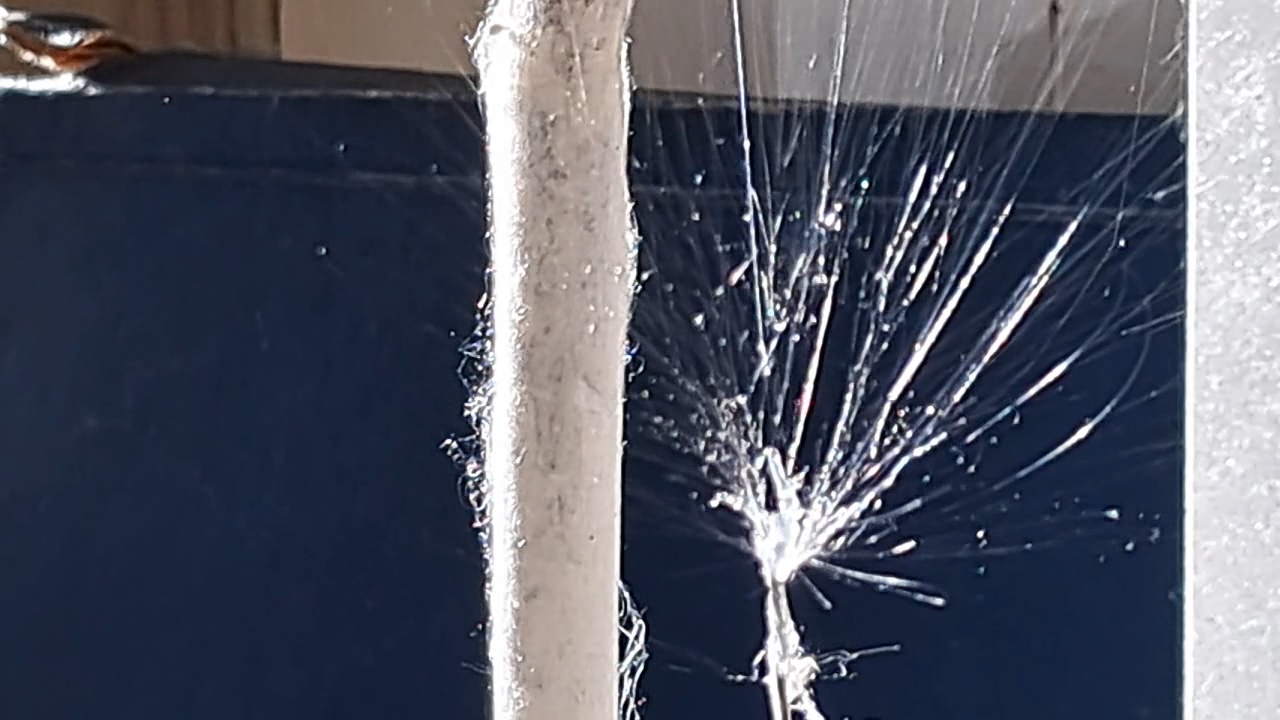
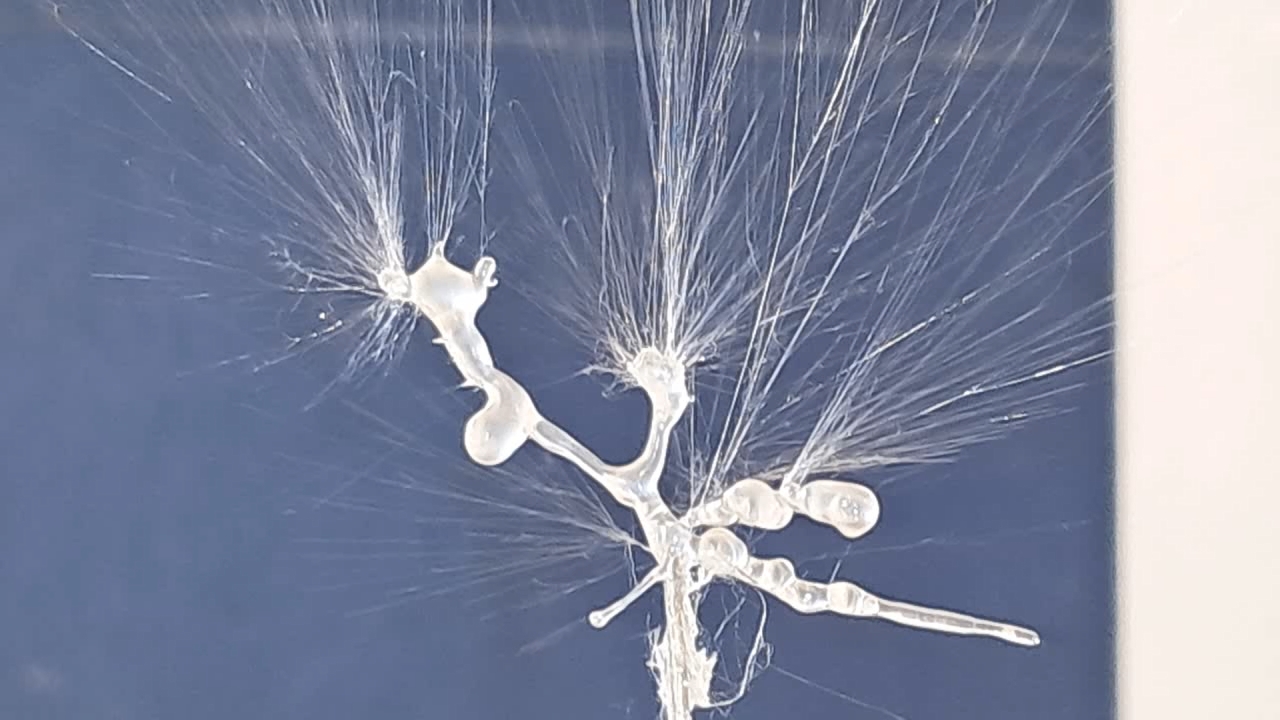
Электроспиннинг: раствор полистирола в этилацетате - Часть 21 There was also another, unheated laboratory whose windows were not exposed to direct sunlight. At the time of these events, the temperature there had dropped to 2°C, and after a couple of weeks of severe frost, it fell to -4°C. This low temperature in the lab had interesting, though not entirely pleasant, consequences, which I will discuss later. Naturally, I conducted the electrospinning experiments in the warm laboratory. I hoped that an air temperature of 20°C would enhance the evaporation of ethyl acetate and eliminate the problem of solution splashing onto the collector surface. Of course, I did not rely on hope alone and also used a heater to further promote solvent evaporation. The bright sunlight made the polystyrene fibers clearly visible. The dynamics of fiber formation and movement became noticeable - something that was impossible to observe under standard lighting. In particular, it was on this occasion that I first noticed that some fibers detached from the needle but did not adhere to the collector or other surrounding surfaces; instead, they were simply carried away by the air flow. The polymer fibers resembled iridescent glass threads or a spider's web in the sunlight - the electrospinning process looked especially beautiful that day. At times, it felt unreal, as if I were watching an AI-generated video rather than observing a real experiment. However, focusing the camera under these conditions proved difficult, so the resulting video was less impressive. If I had had a good high-speed camera, I probably would have been able to capture truly spectacular footage. On the other hand, I would still prefer to buy a high-voltage power supply rather than a camera. As in previous experiments, "beards" and semi-liquid droplets formed and adhered to the needle. These had to be periodically removed using a long plastic stick, which stimulated the formation of new fibers. Unfortunately, it was at this point that splashes often formed and reached the collector. Compared with previous experiments, I was unable to reduce the formation of splashes. In turn, these splashes caused defects in the resulting electrospun polystyrene when they reached the collector. Therefore, ethyl acetate is poorly suited for electrospinning polystyrene, at least under the experimental conditions described here. |

Electrospinning: Solution of Polystyrene in Ethyl Acetate |

|

|

|

|

|

|

|

|

|

|
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|