Chemistry and Chemists № 1 2026
Journal of Chemists-Enthusiasts
| Content | Chemistry experiments - video | Physics experiments - video | Home Page - Chemistry and Chemists |
|
Chemistry and Chemists № 1 2026 Journal of Chemists-Enthusiasts |
Electrospinning - pt.22, 23 Chemist |
|
Having noticed a mistake in the text, allocate it and press Ctrl-Enter
At the same time that I was dissolving polymethyl methacrylate, I also attempted to dissolve another polymer: nitrocellulose. Recently, a colleague had attempted to purchase reactive nitrocellulose for membrane fabrication. When he contacted the supplier, it turned out that the minimum order quantity was 10 kg. Since he did not need such a large amount, he decided not to proceed with the purchase.
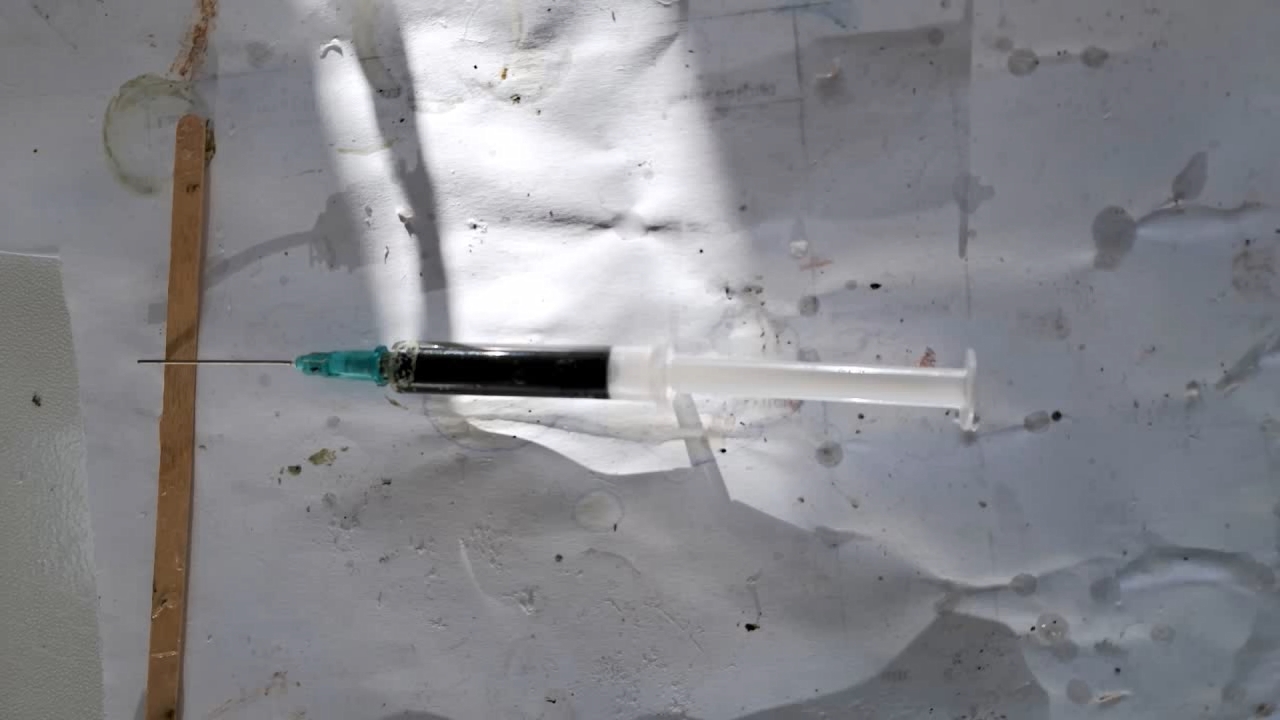
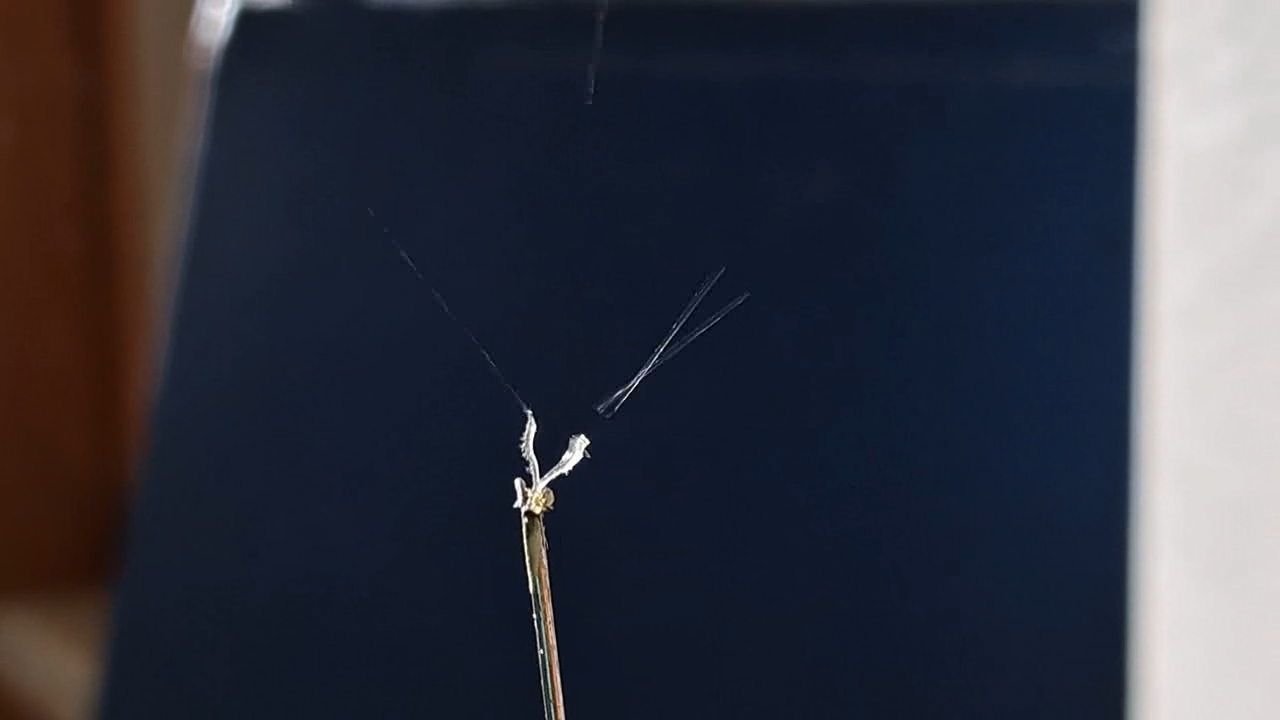
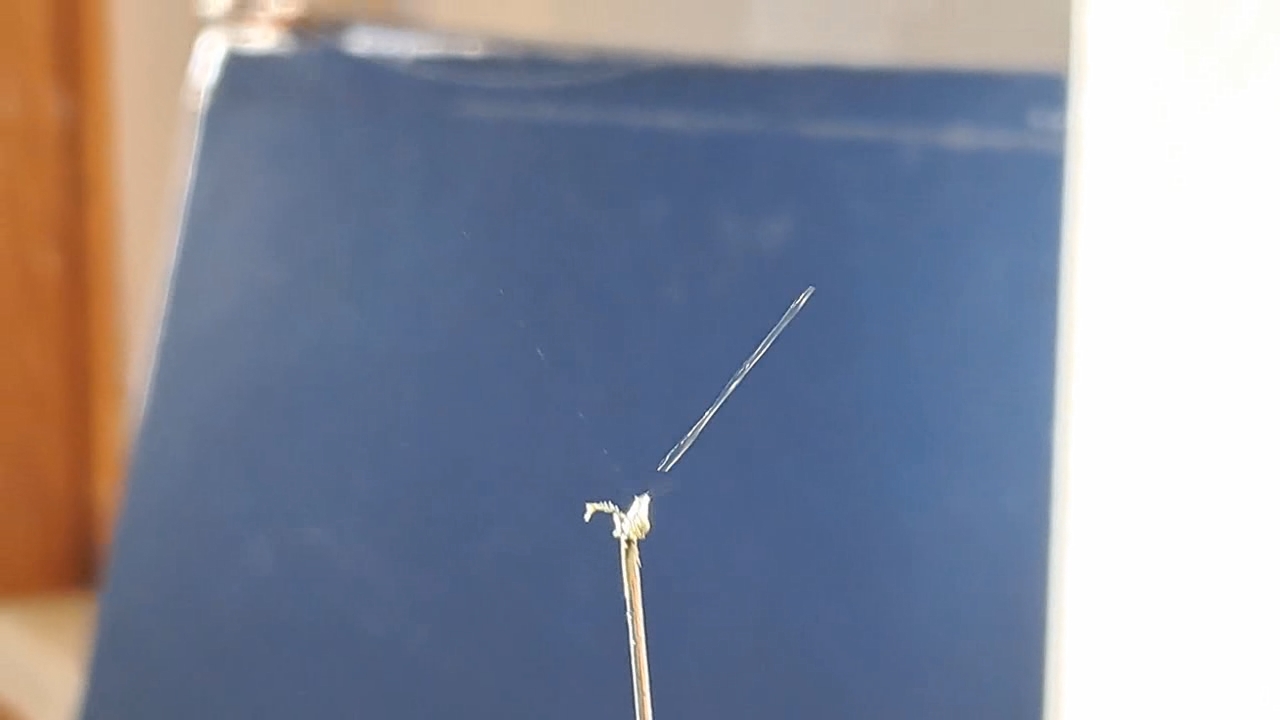
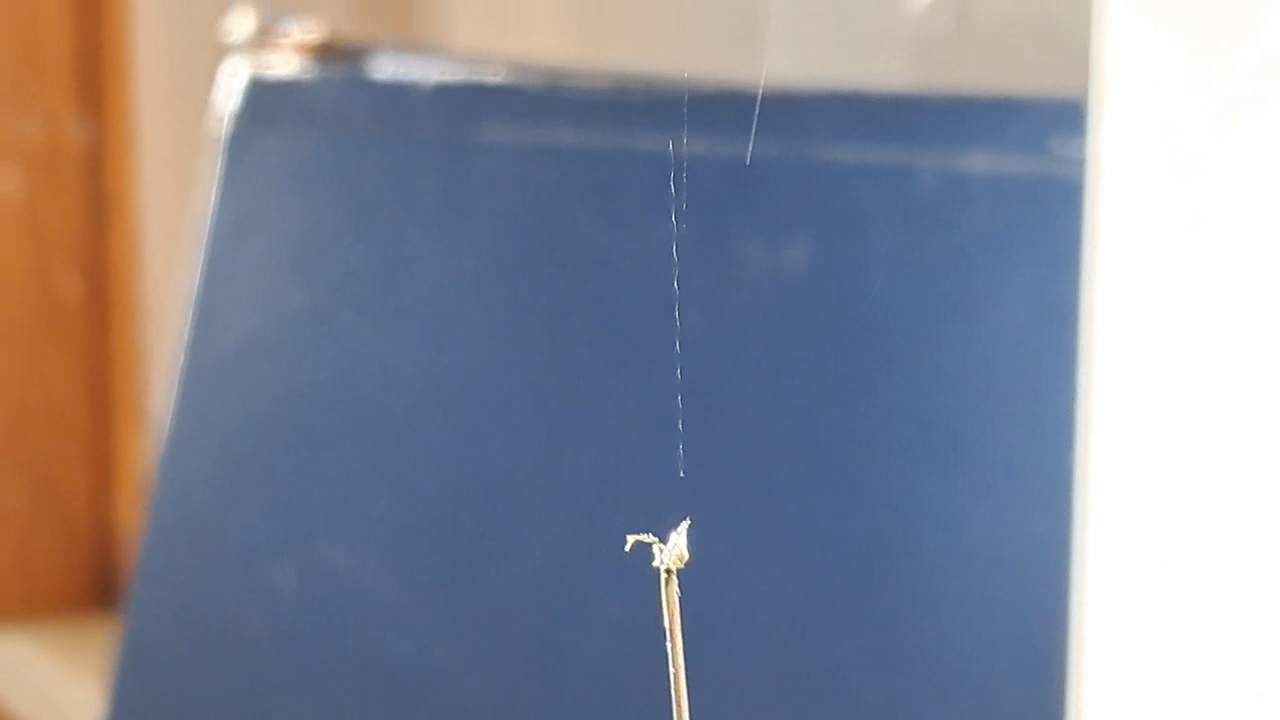
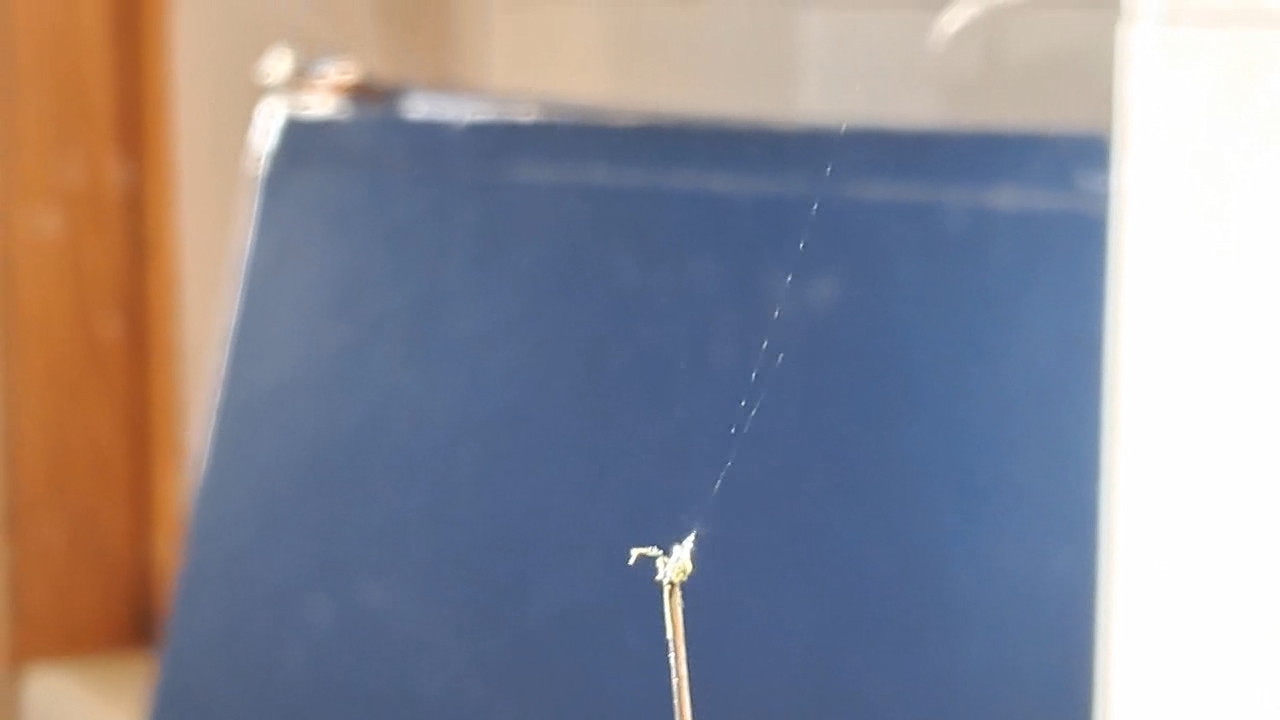
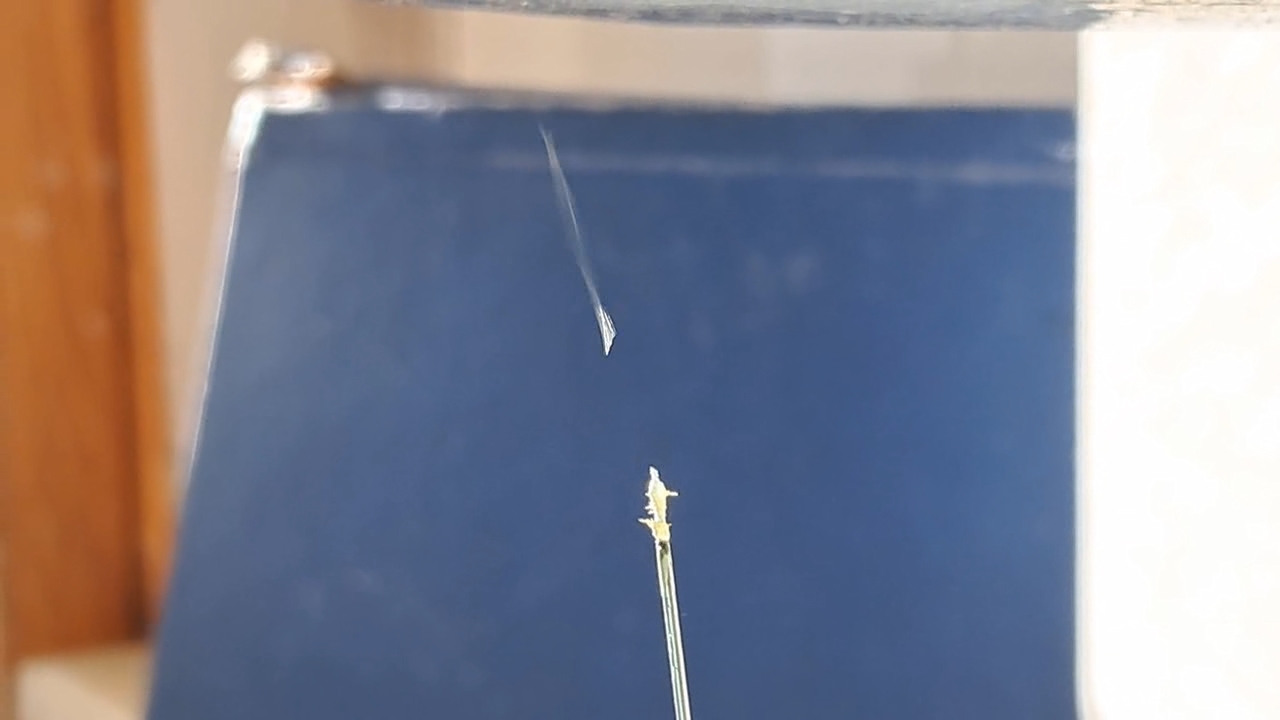
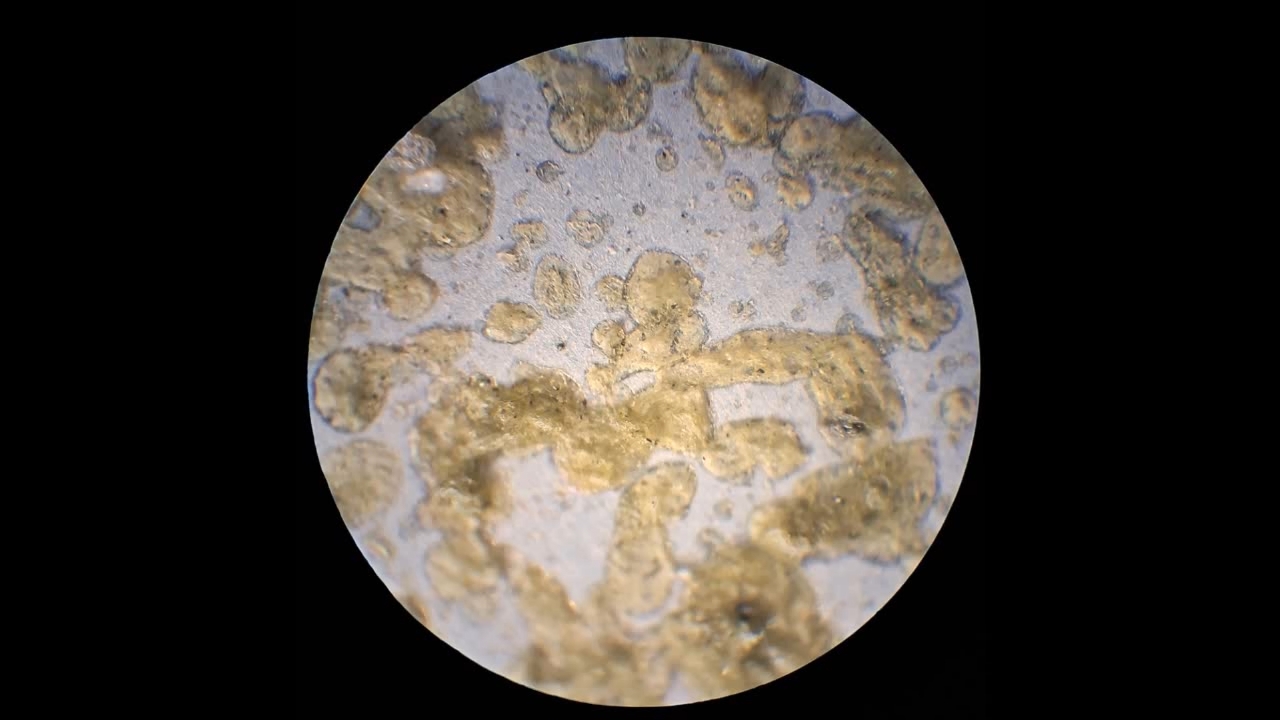
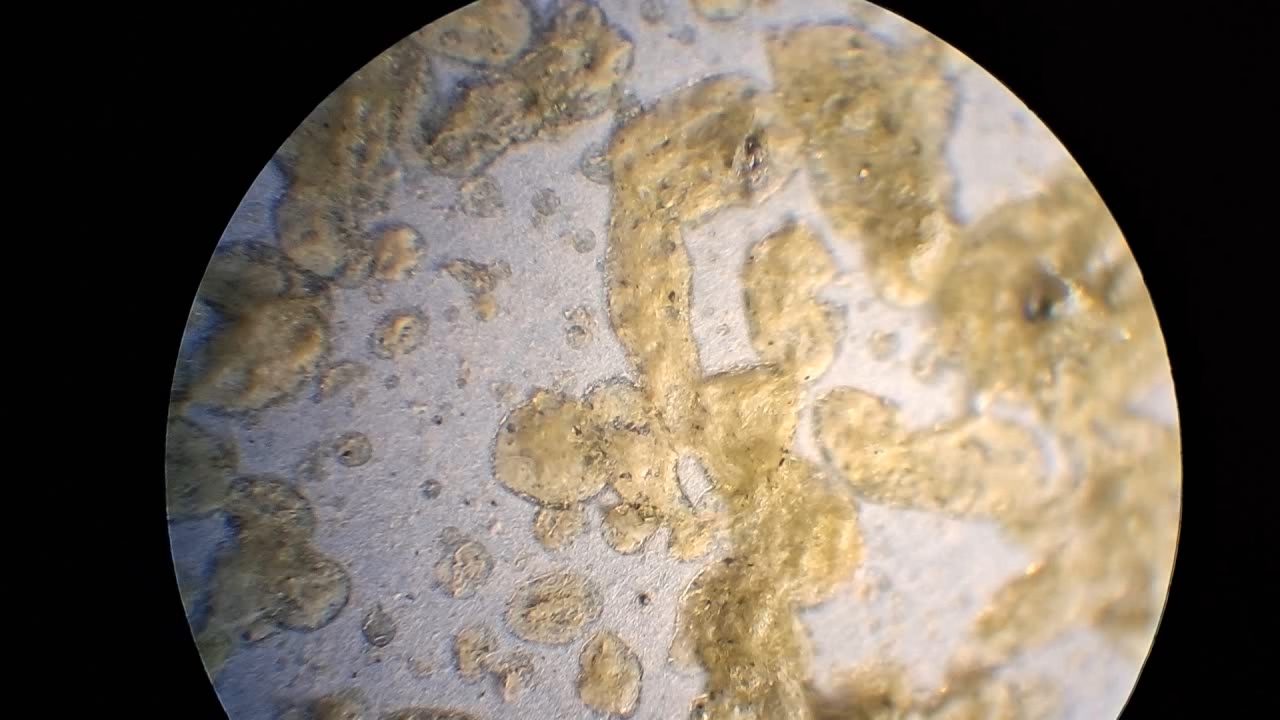
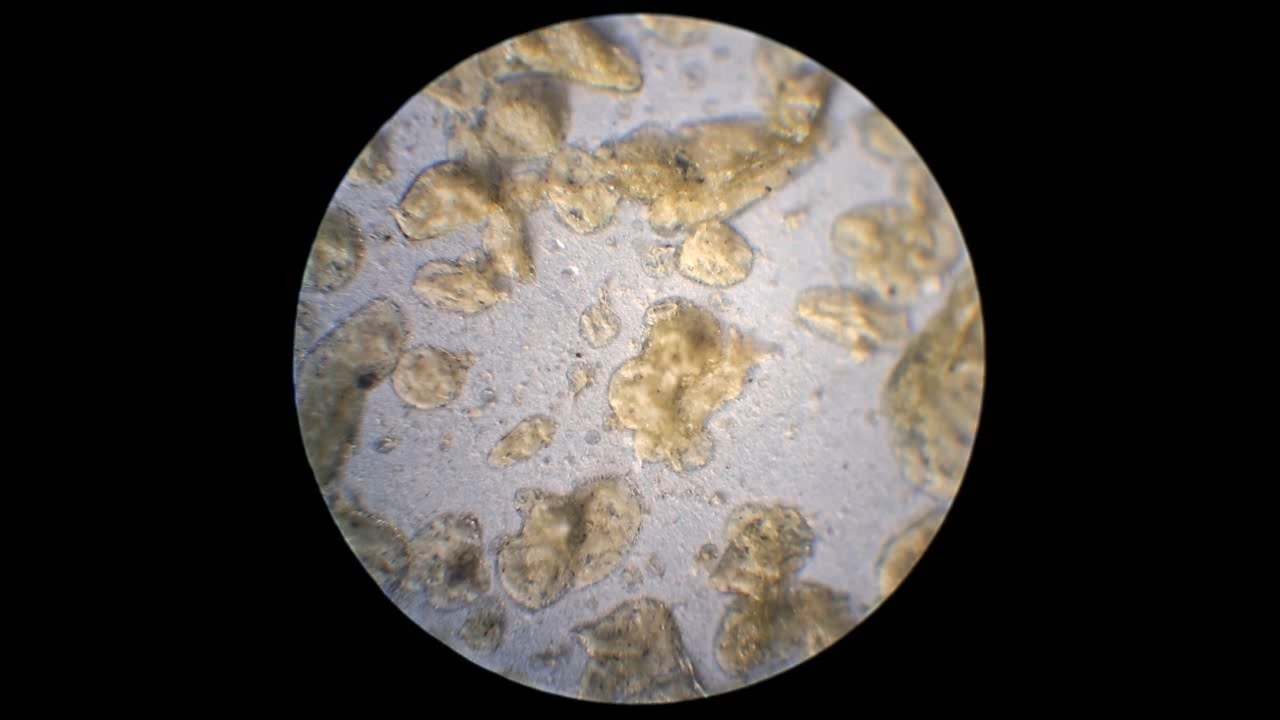
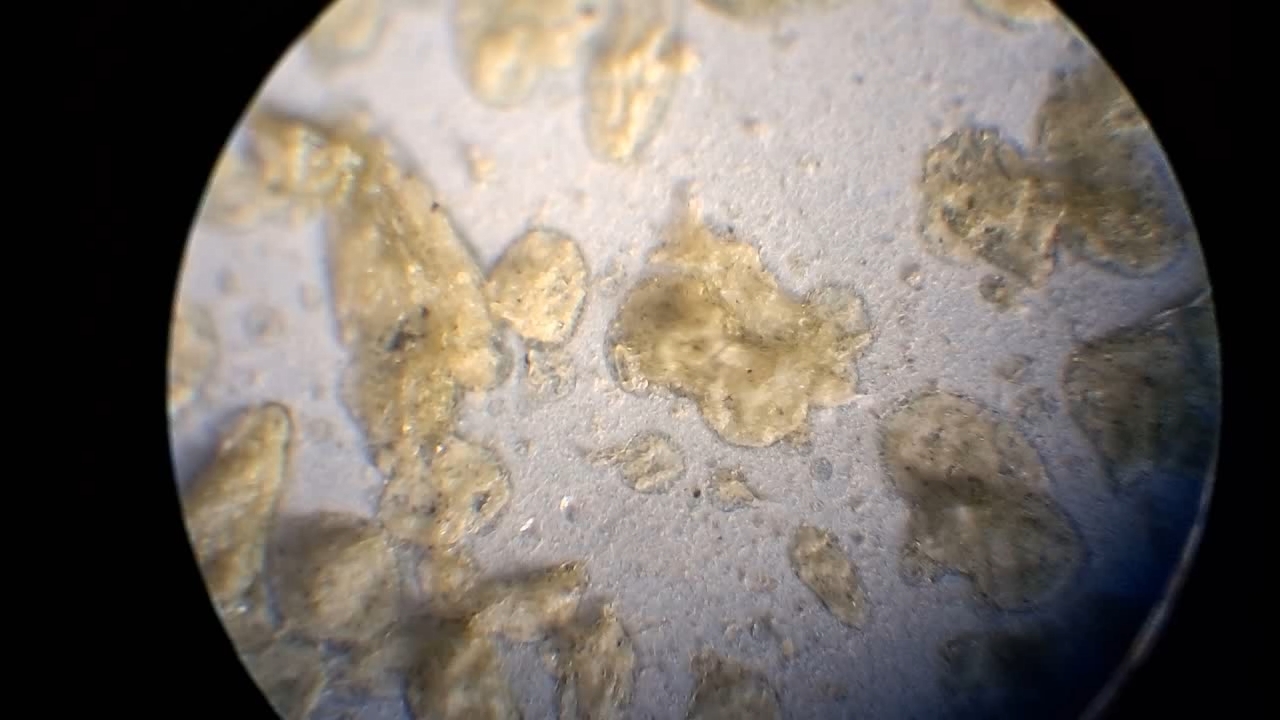
I could have nitrated cotton wool myself, as we did in our youth, but I chose a simpler approach. I had a few grams of "Sokol" ("Falcon") hunting smokeless powder, which consists primarily of nitrocellulose. I decided that, for preliminary experiments, using smokeless powder instead of pure nitrocellulose would be perfectly acceptable. Acetone is a good solvent for this type of smokeless powder. By dissolving smokeless powder in acetone, pyrotechnicians prepare a varnish that is widely used in their work. I was not interested in pyrotechnics and did not have much acetone available, so I decided to look for an alternative solvent. The "Acetone+" substitute dissolves smokeless powder much less effectively than real acetone: dissolution takes a long time, and the resulting solution is more viscous. Interestingly, in the case of polystyrene, the opposite is true - the polymer dissolves well in the substitute but only swells in real acetone. I therefore decided to try methyl acetate as a solvent. I weighed 5.195 g of methyl acetate and 0.520 g of the smokeless powder. After adding the powder to the weighing bottle containing the solvent, the methyl acetate turned yellow. I mixed the contents, sealed the bottle tightly, and stirred it occasionally. The nitrocellulose gradually swelled and dissolved, forming a green solution with relatively low viscosity. At the same time, I mixed the same amounts of the smokeless powder and the "Acetone+" solvent. By the next day, a viscous liquid had formed. I added another 3 g of the solvent in portions; the viscosity decreased, but the solution still remained more viscous than the nitrocellulose solution in methyl acetate. Since the nitrocellulose solution in methyl acetate had a significantly lower viscosity, I decided to use it for electrospinning. I drew the solution into a syringe, fixed the syringe in the syringe pump, turned on the high voltage, and started the solution feed. "Protrusions" formed at the tip of the needle, actively ejecting jets as well as irregularly shaped "pieces" of the nitrocellulose solution. The appearance of the process was unlike anything I had observed in my numerous experiments with polystyrene or in my few experiments with polymethyl methacrylate. I hoped that the jets of solution would transform into fibers. I even imagined producing something resembling nitrated cotton wool, which burns in the palm of the hand with a bright flash but does not injure the skin. Electrospun nitrocellulose could also, in principle, be subjected to alkaline hydrolysis to yield cellulose. Unfortunately, even during the experiment it became clear that, although a coating was forming on the collector, it was most likely not fibrous. To confirm this, I stopped the process and examined the collector under a microscope. Instead of fibers, I observed hardened lumps of nitrocellulose. I returned the collector to its position, reduced the distance between the electrodes as much as possible, and turned the voltage back on. A miniature lightning bolt flashed between the needle and the collector, accompanied by a loud sound. Curiously, such a powerful spark did not ignite the nitrocellulose coating the collector. I immediately turned off the power and measured the distance between the electrodes - it turned out to be 35 mm. Later, I called the physicist who had previously worked on electrospinning together with my chemist colleague and told him what had happened. It turned out that, in his experiments, the distance between the electrodes was at least 120 mm. It was clear that either the voltage between the electrodes was insufficient to form nitrocellulose fibers, or that nitrocellulose simply does not form fibers under these conditions. I stopped the process. The experiments with both polymethyl methacrylate and nitrocellulose had failed. There was only one day left before the New Year. I had no new ideas. The only one I tried was dissolving polymethyl methacrylate in tetrahydrofuran and the "Acetone+" solvent, hoping this would yield a solution with lower viscosity than in the case of dichloromethane. |

Electrospinning: Nitrocellulose |

|

|

|

|

|

|

|

|

|
|

|
|

|

|

|

|

|

|

|

|

|

|

|

|
|
|
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|

|

|

|

|

|

|

|

|

|

|
|
|
|
|