Chemistry and Chemists № 1 2026
Journal of Chemists-Enthusiasts
| Content | Chemistry experiments - video | Physics experiments - video | Home Page - Chemistry and Chemists |
|
Chemistry and Chemists № 1 2026 Journal of Chemists-Enthusiasts |
Electrospinning - pt.24, 25 Chemist |
|
Having noticed a mistake in the text, allocate it and press Ctrl-Enter
New Year's Day in the Laboratory. Dissolving Polystyrene in Dimethylformamide - Part 24
When you don't know how to solve an experimental problem, you need time to think. Often, ideas appear on their own, without any apparent effort. Sometimes you don't even realize how a new and promising idea came to you. It's good to have colleagues you can consult. So, on New Year's Day, I decided to visit the chemist I had worked with: we could discuss the problem, and perhaps he would suggest something. Even if no new ideas emerged, we would simply sit together and talk. I didn't tell him about my visit, confident that he would stay home for the holiday. What could possibly go wrong?
День Нового года в лаборатории. Растворение полистирола в диметилформамиде - Часть 24 I entered the building; the power was out - the elevators weren't working. I started climbing the stairs to his apartment on the 16th floor. About halfway up, I ran into… my colleague, who was coming down. "Hi, where are you going?" "To work." "It's New Year's!" "There's no power, and there won't be for many hours - there's no point in staying home. At work, according to the schedule, the power should be on until evening." As I write these lines, power outage schedules no longer exist. The bombings have severely damaged the power grid. Electricity can go out at any time, and it's impossible to predict when it will return. At the time of these events, however, the schedules were still in place, although they weren't always followed. I hesitated, wondering whether to join my colleague and go to work or go up to his apartment and spend the holiday with his family and cat. His family includes two other chemists, but they weren't familiar with the details of our experiments. Should I go to the institute on New Year's Day? Clearly, no one would be there except the security guard - he would be surprised. But the main thing that held me back was that I had completed my current experiments, and the most recent ones had been unsuccessful. I had no idea what I would do in the laboratory. After some thought, I joined my colleague, although I was quite annoyed with him. We went to work. It was sunny outside, but bitterly cold. On the way, I was thinking about which experiment to begin. I had recently prepared PMMA solutions in tetrahydrofuran and in the "Acetone+" solvent. Both solutions turned out to be too viscous and unsuitable for electrospinning. I could try electrospinning one of them, although a negative result was almost guaranteed. Another option was to dissolve expanded polystyrene in dimethylformamide (DMF) and then attempt electrospinning. According to reference data, polystyrene dissolves well in DMF. The idea of using this solution for electrospinning had been on my mind for a long time. However, DMF has a very high boiling point - the highest of all the solvents I had used up to that point. When I electrospun polyvinylidene difluoride, the DMF did not evaporate completely, resulting in a viscous, slime-like mass rather than fibers. I hadn't obtained any positive results with DMF before. Electrospinning polystyrene with other solvents had worked reasonably well, so there seemed to be little point in setting up an experiment that was almost certain to fail. We arrived at the institute. There wasn't a single footprint in the snow near the entrance - none of the employees had come in. The security guard was very surprised but didn't complain, since New Year's Day was officially a working day. I went to the laboratory. In the end, I decided to try electrospinning the PMMA solution in the "Acetone+" solvent. I drew the solution into a syringe, secured it in the setup, turned on the high voltage and the syringe pump - and nothing happened. No aerosol formed, and no fibers appeared. The electrostatic force couldn't even detach a semi-solidified drop of solution from the needle. This outcome had been obvious even before the experiment began. I then decided to dissolve expanded polystyrene in dimethylformamide. I was fairly certain that electrospinning this solution would also be unsuccessful. Still, I comforted myself with the thought that the dissolution process could at least be filmed and described in a popular science article. I weighed 9.700 g of DMF and 1.525 g of expanded polystyrene and added the polymer pieces to the solvent. Gas was released, and the dissolution process was noticeably slower than with all the solvents I had used previously: "Acetone+," ethyl acetate, dichloromethane, and tetrahydrofuran. I had to stir the contents of the bottle more frequently. A relatively large amount of foam formed. Most importantly, the polystyrene did dissolve. As with the other solvents, the last pieces dissolved rather slowly. The result was a yellowish, turbid solution with a polymer concentration of 13.58%. To my surprise, the viscosity of the solution was quite low. |
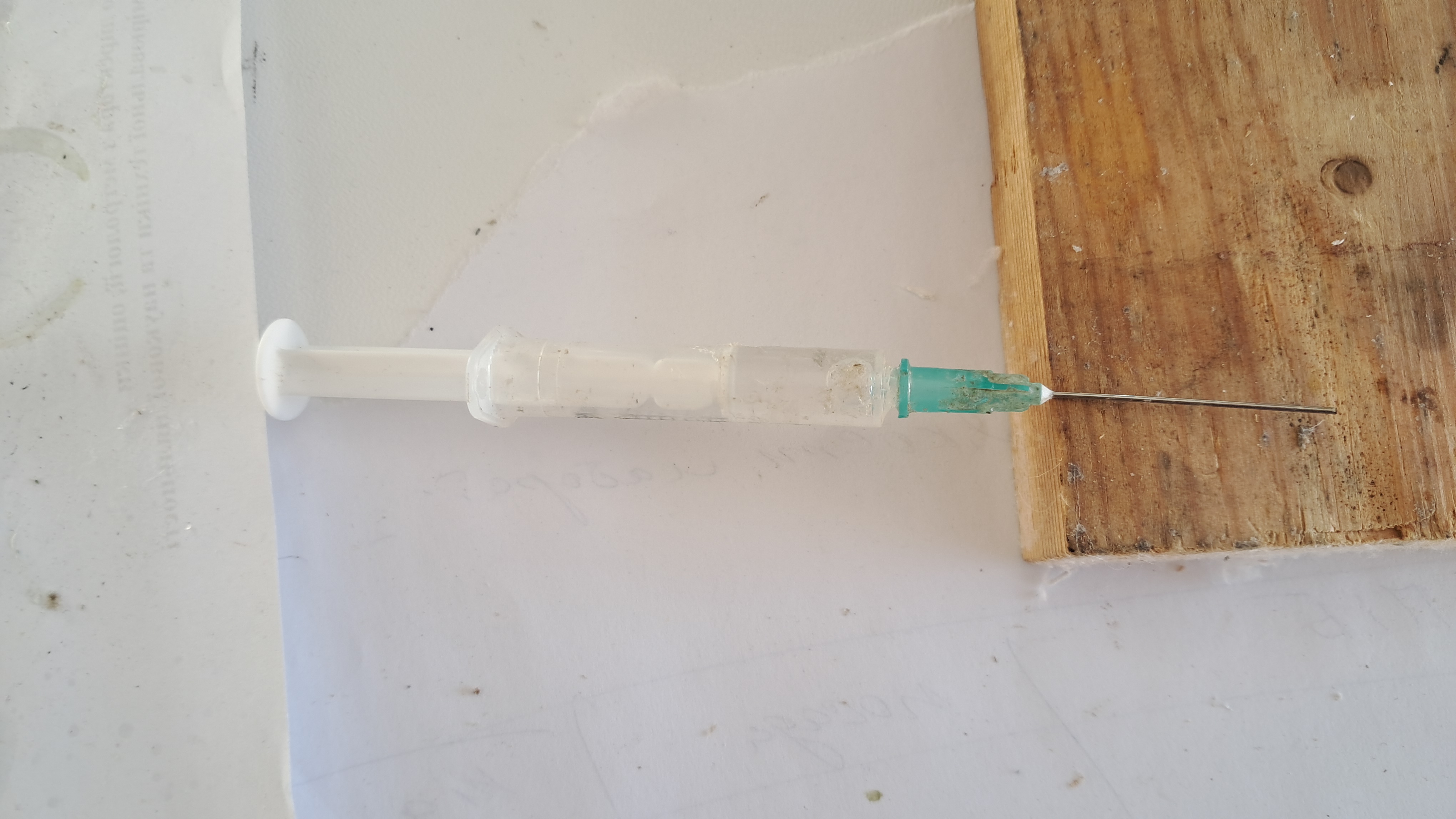
Electrospinning of the solution of polymethyl methacrylate (PMMA) in surrogate acetone |

|

New Year's Day in the Laboratory. Dissolving Polystyrene in Dimethylformamide |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|