Chemistry and Chemists № 1 2026
Journal of Chemists-Enthusiasts
| Content | Chemistry experiments - video | Physics experiments - video | Home Page - Chemistry and Chemists |
|
Chemistry and Chemists № 1 2026 Journal of Chemists-Enthusiasts |
Electrospinning - pt.28, 29, 30 Chemist |
|
Having noticed a mistake in the text, allocate it and press Ctrl-Enter
Electrospinning: Solutions of Polystyrene in Dimethylformamide - Part 28
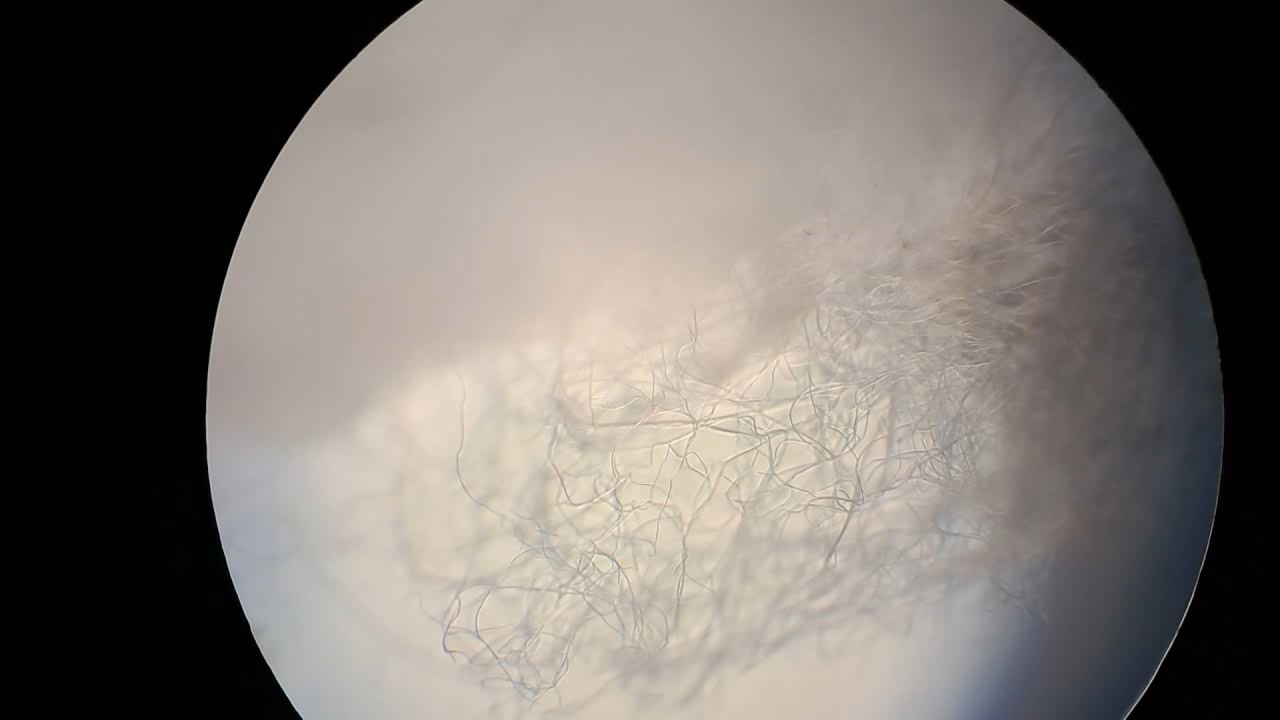
In my first experiment with polystyrene and DMF, I prepared a moderately concentrated solution (13.58%), as I was unsure whether the polymer would dissolve at all. I was even more doubtful that electrospinning this solution would produce fibrous material. Since a positive result was unexpectedly obtained, I decided to increase the polystyrene concentration.
Электроспиннинг: раствор полистирола в диметилформамиде - Часть 28 I gradually added 2.560 g of expanded polystyrene to 10.085 g of dimethylformamide. The polymer dissolved, forming a white, turbid solution with a concentration of 20.2%. To my surprise, the viscosity of the liquid remained relatively low. I used the resulting solution for electrospinning. Visually, the process was similar to the previous experiment using a 13.58% polystyrene solution. A cotton wool-like coating formed on the collector, and fibers were visible under a microscope. No splashing of the solution was observed this time. After using about 2 mL of the solution, I stopped the experiment, intending to further increase the polymer concentration. |

Electrospinning: Solutions of Polystyrene in Dimethylformamide |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|

|
|
Having noticed a mistake in the text, allocate it and press Ctrl-Enter
Electrospinning: Solutions of Polystyrene in Dimethylformamide - Part 29
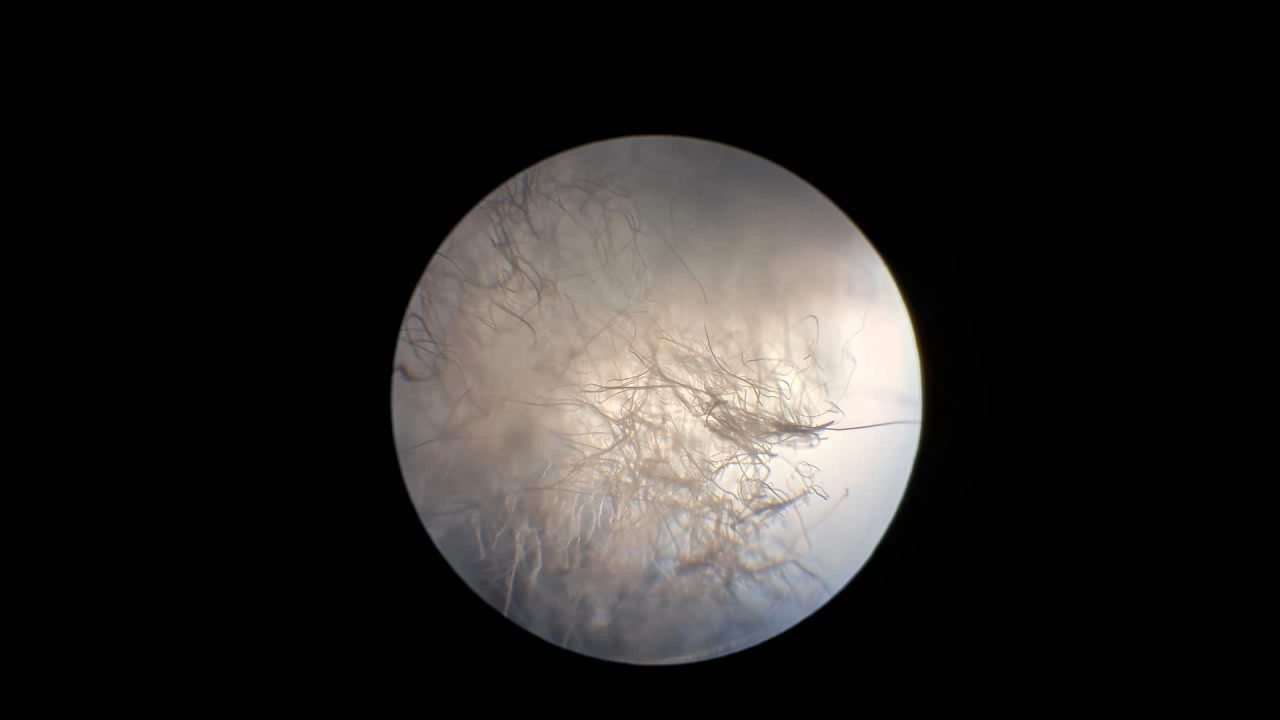
I prepared a new polystyrene solution by weighing 2.025 g of my existing 20.2% polystyrene solution and adding 0.225 g of expanded polystyrene. I was unsure whether the polymer would dissolve and whether the resulting solution would be suitable for electrospinning, so I deliberately used minimal amounts of the substances. Upon stirring, the polymer gradually dissolved, forming a 28.2% solution with a viscosity that proved to be perfectly acceptable.
Электроспиннинг: раствор полистирола в диметилформамиде - Часть 29 I began electrospinning. It was a frosty, sunny day, and bright rays of sunlight fell on the needle from the side. The result was unexpected: streams of solution escaping from the needle resembled sparkling whips. These "whips" then disintegrated into bundles of streams that transformed into threads. Under different lighting conditions, this process remained invisible. However, the "formation of cotton wool in the air" was almost not observed. A characteristic white coating of electrospun polystyrene formed on the collector. When the solution ran out, I decided to prepare a new solution and continue the process without separating the already formed coating from the collector. |

Electrospinning: Solutions of Polystyrene in Dimethylformamide |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
Having noticed a mistake in the text, allocate it and press Ctrl-Enter
Electrospinning: Solutions of Polystyrene in Dimethylformamide - Part 30
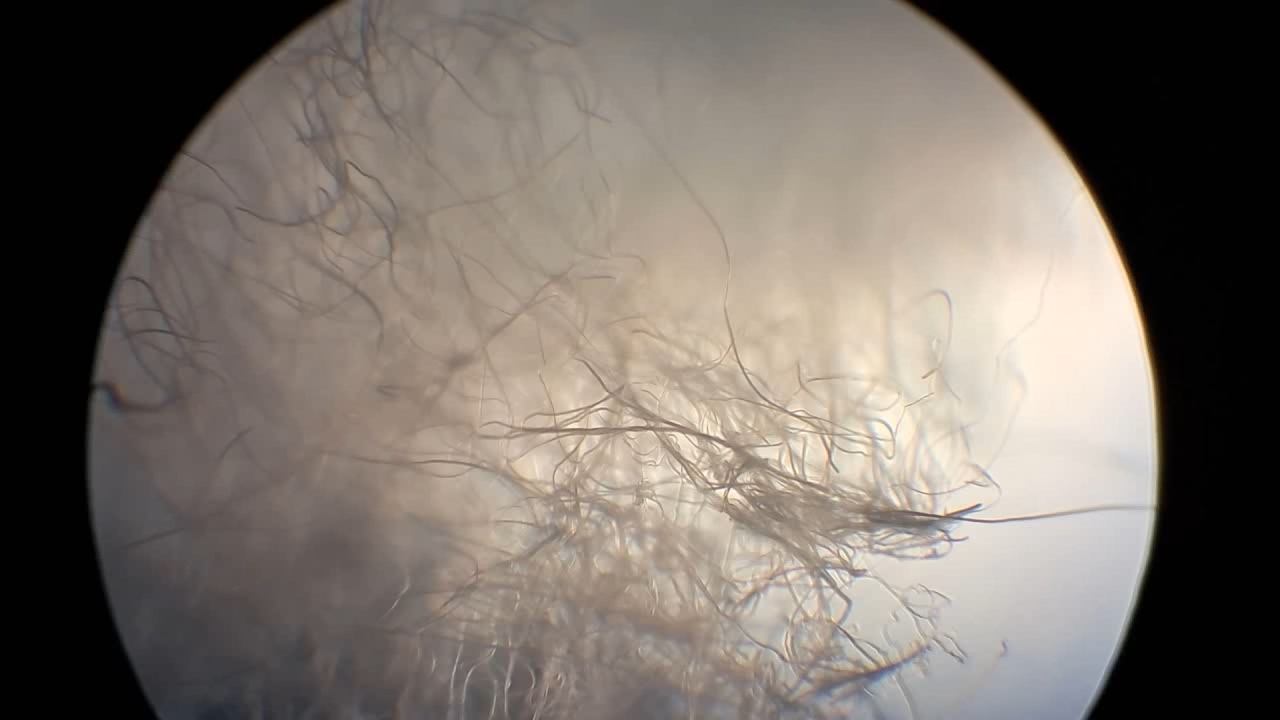
To continue electrospinning, I needed to prepare a new polystyrene solution in DMF with a high polymer concentration. It turned out that, after numerous experiments, I had used up all the glass bottles available in the laboratory. As a result, I had to prepare the solution in a PET bottle, even though I was not certain whether this polymer was resistant to DMF.
Электроспиннинг: раствор полистирола в диметилформамиде - Часть 30 I weighed out 10.120 g of dimethylformamide and 4.120 g of expanded polystyrene and gradually added the polymer to the solvent. Large pieces of polystyrene became highly electrified during breakage and stuck to my gloves. Under calmer circumstances, I could have filmed a video demonstrating electrostatic forces, but I had a different objective at the time. Although the volume of expanded polystyrene was much greater than that of the DMF, the polymer dissolved. A white solution with a polystyrene concentration of 28.9% and moderate viscosity formed. I filled a syringe with the solution and continued electrospinning. This time, I was able to observe the "formation of cotton wool in the air": a fibrous material was literally forming before my eyes. At a certain point, fiber formation suddenly stopped, even though some polystyrene solution still remained in the syringe. I assumed that the solution had hardened inside the needle, blocking the flow. The syringe pump is designed for use with 20 ml syringes. When the plunger movement is blocked, or when the plunger reaches its maximum upper position (that is, when all the solution has been expelled), an audible alarm should sound. However, no alarm was triggered. This did not surprise me, as I had modified the pump's standard operating configuration. Instead of 20 ml syringes, I use 2 ml syringes together with a special rod that transfers force from the pump to the syringe plunger. When using 2 ml syringes, the alarm usually does not sound. I turned off the high voltage, removed the syringe from the setup, and discovered that the rod was already in its maximum upper position. This position corresponds to the fully depressed syringe plunger - that is, to a state in which all the solution should have been expelled. However, some solution still remained in the syringe. The plunger movement had been blocked because the needle was clogged. The conclusion was clear: under pressure, the thin plastic plunger rod had begun to bend, allowing the pump rod to reach its uppermost position before the syringe was actually empty. With 20 ml syringes, the plunger rod is much thicker and more rigid, which reliably triggers the alarm. I quickly replaced the needle and resumed electrospinning. Eventually, the solution in the syringe was completely exhausted, and the collector became coated with a thick layer of cotton-like material. It is worth noting that the solution used for electrospinning consisted of nearly one-third polymer by weight. |

Electrospinning: Solutions of Polystyrene in Dimethylformamide |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
|