Chemistry and Chemists № 1 2026
Journal of Chemists-Enthusiasts
| Content | Chemistry experiments - video | Physics experiments - video | Home Page - Chemistry and Chemists |
|
Chemistry and Chemists № 1 2026 Journal of Chemists-Enthusiasts |
Electrospinning - pt.31, 32 Chemist |
|
Having noticed a mistake in the text, allocate it and press Ctrl-Enter
Electrospinning: Solution of Polystyrene in Dimethyl Sulfoxide - Part 31
Despite the relatively high boiling point of the solvent, polystyrene solutions in DMF turned out to be excellent for electrospinning. A plausible explanation for this result is the relatively high electrical conductivity of DMF. Another liquid capable of dissolving polystyrene - dimethyl sulfoxide (DMSO) - has even higher electrical conductivity.
Электроспиннинг: раствор полистирола в диметилсульфоксиде - Часть 31 Unfortunately, DMSO has an even higher boiling point than DMF (189°C and 153°C, respectively). In addition, I found in literature that polystyrene dissolves more slowly in DMSO than, for example, in dichloromethane or tetrahydrofuran. Despite these drawbacks, I decided to try electrospinning a polystyrene solution in DMSO. I had greatly enjoyed my recent experiments with polystyrene solutions in DMF - not only from a scientific standpoint, but also from an aesthetic one - and I hoped that using DMSO might yield even better results. When my chemist colleague learned that I was planning to use dimethyl sulfoxide, he practically started yelling at me: "DMSO penetrates the skin very easily - you'll poison yourself!" My colleague is usually calm; this reaction was unusual for him. I replied calmly: "DMSO is a non-toxic solvent; it's widely used in medicine." "If something toxic is dissolved in it, DMSO will carry the poison through your skin!" "Polystyrene is non-toxic; there are no other substances in the solution." "But what if the solvent contains..." "Or maybe an asteroid will hit our lab in a minute. Please don't get on my nerves." I weighed out 5.050 g of DMSO and 0.720 g of expanded polystyrene and added several pieces of the polymer to the solvent. No gas evolution or other visible signs of dissolution were observed. In acetone, expanded polystyrene rapidly loses gas and shrinks in volume, even though it does not dissolve. In contrast, with dimethyl sulfoxide, no external changes were apparent. I sealed the weighing bottle and left it overnight. In the morning, I found that some of the DMSO had leaked out of the bottle, but the expanded polystyrene had not dissolved. The solvent had an unpleasant odor, reminiscent of radish or garlic. I enjoy both radish and garlic, but the smell of DMSO was frankly nauseating. Chemically pure DMSO is odorless; the unpleasant smell is caused by the presence of dimethyl sulfide (DMS). Many organic sulfur compounds have extremely unpleasant odors. According to the literature, after ingestion of DMSO, a person's breath and skin may acquire a similar odor, which is caused by the reduction of DMSO to DMS during metabolism. The solubility of a polymer strongly depends on its molecular weight, so it did not surprise me that the expanded polystyrene failed to dissolve in DMSO. I had long suspected that the polystyrene sample used in my experiments had a high molecular weight. I heated the mixture of expanded polystyrene and DMSO in a boiling water bath. To my delight, the polystyrene dissolved. I added additional pieces of expanded polystyrene. At first, the polymer continued to dissolve, but eventually the process stopped - I was unable to dissolve all of the weighed material. As I was conducting other experiments at the same time, I was unable to film the process. After removing the weighing bottle from the water bath and allowing it to cool to room temperature, the undissolved polystyrene formed a hard lump. I drew the liquid phase into a syringe and mounted it in the setup. Concerned about incomplete evaporation of DMSO during electrospinning, I placed a heater near the setup. I then turned on the high voltage and the solution supply. It quickly became apparent that, instead of a solid fibrous material, the collector was being coated with liquid. The solvent did not evaporate completely during electrospinning. I stopped the process and examined the collector under a microscope. No fibers were observed, and I nearly smeared the microscope objective with the liquid coating the electrode. I considered reheating the syringe containing the solution in a water bath and continuing electrospinning, but the power unexpectedly went out. I had to pack up in the dark and go home. The next day, I decided not to continue the experiment. After heating in a water bath, the small 2 ml syringe would have cooled rapidly, and the collector would once again have been coated with liquid rather than polystyrene fibers. Electrospinning of a polystyrene solution in DMSO had clearly failed, although some of the solution remained. I decided to perform a simple chemical demonstration. Dimethyl sulfoxide is highly soluble in water, whereas polystyrene is insoluble. Therefore, when a solution of polystyrene in DMSO is poured into water, the polystyrene should precipitate, forming a white suspension. When I poured the solution into a glass of water and stirred it, the water remained clear. A few tiny pieces of polystyrene were floating in the glass, but they could have been carried in with the liquid. Conclusion: dimethyl sulfoxide contained little or no dissolved polystyrene at room temperature. The polymer that dissolved upon heating precipitated as a solid phase when the solution cooled. It turned out that I had been attempting to electrospin… the pure solvent. |

Electrospinning: Solution of Polystyrene in Dimethyl Sulfoxide |

|

|

|

|

|

|

|

|

|

|

The next day |

Some of the DMSO had leaked out of the bottle |

|

|

|

|

|

|

|

After the water bath |

|

|

|

|

|

The power unexpectedly went out |

|

A glass of water |

When I poured the solution into the glass of water and stirred it, the water remained clear |

|

Methylene chloride and PVC |

|

|

|

|

|

DMF and PVC |

|

THF and PVC |

|

|
PMMA in DMF (left), PVC in DMF (center), PVC in THF (right) |

|

|

|

|

PVC in THF |

|

PVC in DMF |

|

|

|

Electrospinning: Polyvinyl Chloride in THF |

|

|

|

|

|

|

|

|

|
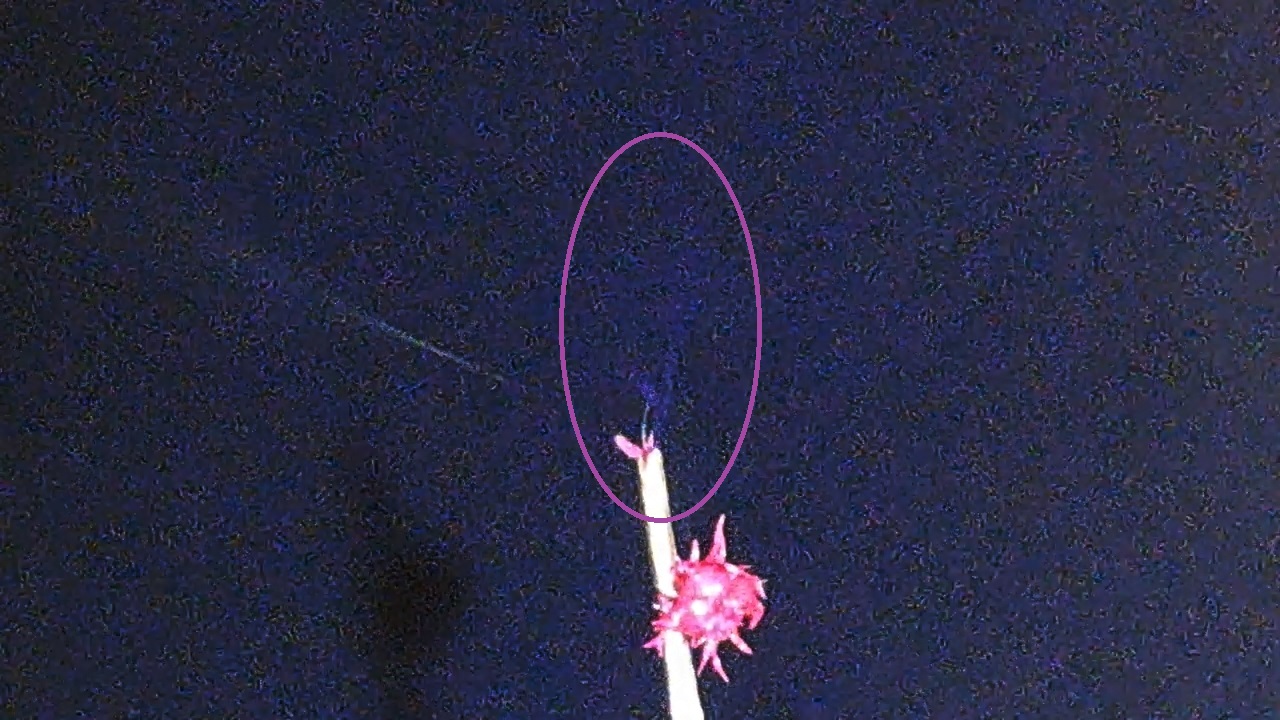
Corona discharge |

|

|

|

|

|

|

PVC in DMF |

|

Electrospinning: Polyvinyl Chloride in DMF |

|

|

|
|
Комментарии
К1
Добавлением воды в ацетоновый раствор полистирола, готовят эталоны для гель-проникающей хроматографии: добавили каплю воды, отцентрифугировали, снова добавили и т.д. В результате получается набор образцов полимера с достаточно точно известной молекулярной массой. Ими можно калибровать хроматограф.
К1-1 Мой напарник (один из трех героев рассказа) предложил, что полистирол можно разогнать по массам на колонке. В ответ я поинтересовался, не ты ли собираешься это сделать? На чем тема как-то заглохла. Много лет назад в другом институте, он делил по молекулярным массам органические вещества природных вод на колонках с сефадексом (реки и озера), но сейчас не хочет ничего делать. С ужасом пытаюсь оценить, насколько моего желания что-то делать хватит? К2 Аптечный димексид может содержать влагу. К2-1 Это был не аптечный. В свое время я его использовал для эксперимента по свечению люминола, который окислялся воздухом. Добавлял карбид кальция для осушки - выделения ацетилена не наблюдалось. Link: Luminescence of Luminol [Glowing of Luminol Solution] Литровая бутылка на фото в двух этих статьях - та же (с разницей в 15 лет): 

К3 Изоляция проводов практически всегда содержит пластификатор. В СССР использовали дибутилфталат. В моей квартире проводка служит лет 40. А китайцы пластифицируют чем попало, провода или дубеют, или раскисают. Без пластификатора - винипласт. К3-1 Согласно литературе, чистый PVC хрупкий, изоляция - обязана быть пластичной, поэтому наличие пластификатора - само собой разумеется, но я использовал, что было. |