Chemistry and Chemists № 1 2026
Journal of Chemists-Enthusiasts
| Content | Chemistry experiments - video | Physics experiments - video | Home Page - Chemistry and Chemists |
|
Chemistry and Chemists № 1 2026 Journal of Chemists-Enthusiasts |
Electrospinning - pt.33, 34 Chemist |
|
Having noticed a mistake in the text, allocate it and press Ctrl-Enter
Electrospinning: Polyvinyl Chloride and Polystyrene - Part 33
There are many reasons why, during polymer electrospinning, an aerosol forms instead of fibers. One of them is insufficient solution viscosity. Solution viscosity typically increases with increasing polymer concentration. Therefore, I needed to prepare a PVC solution in THF or DMF at a higher concentration than in the previous experiment.
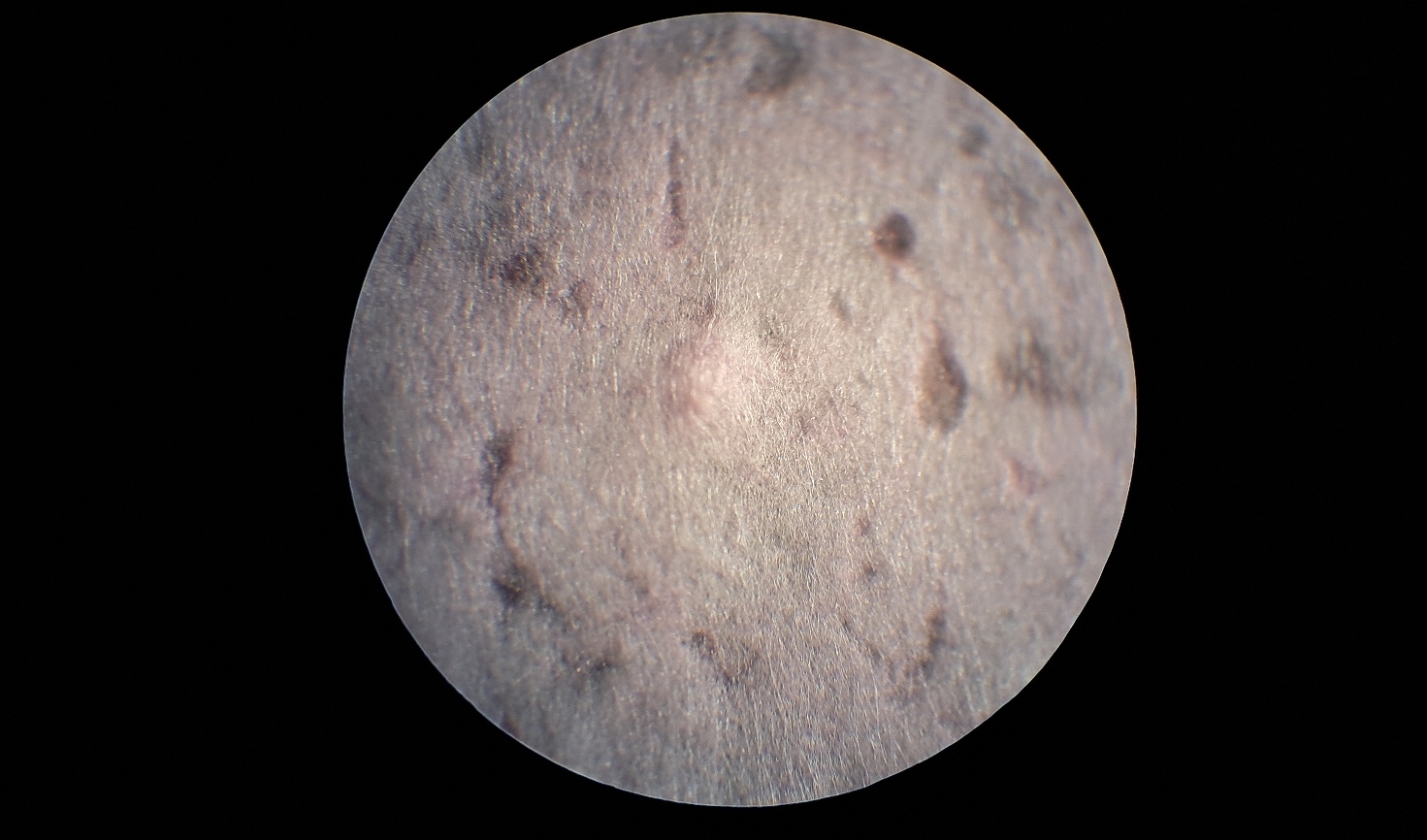
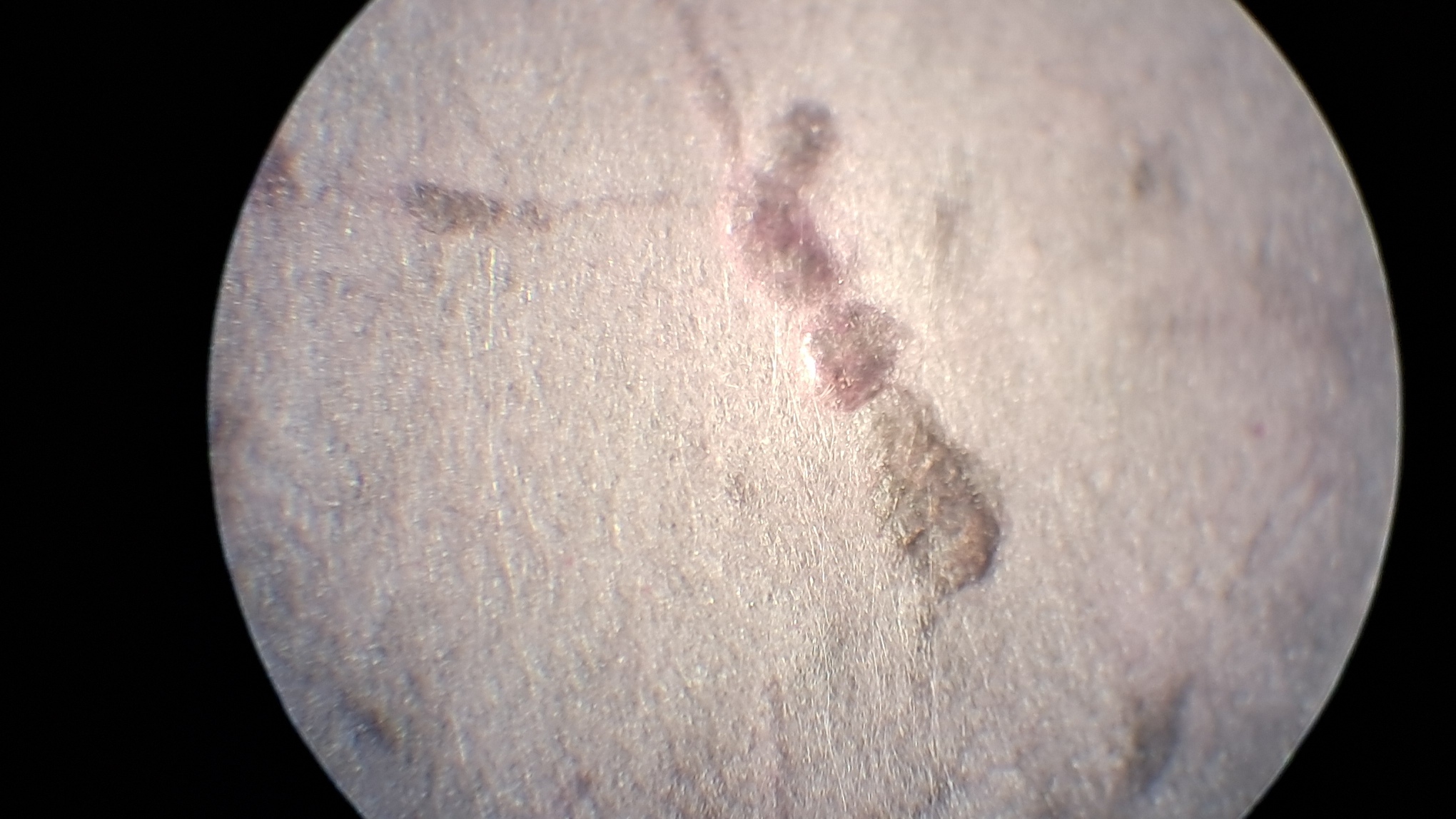
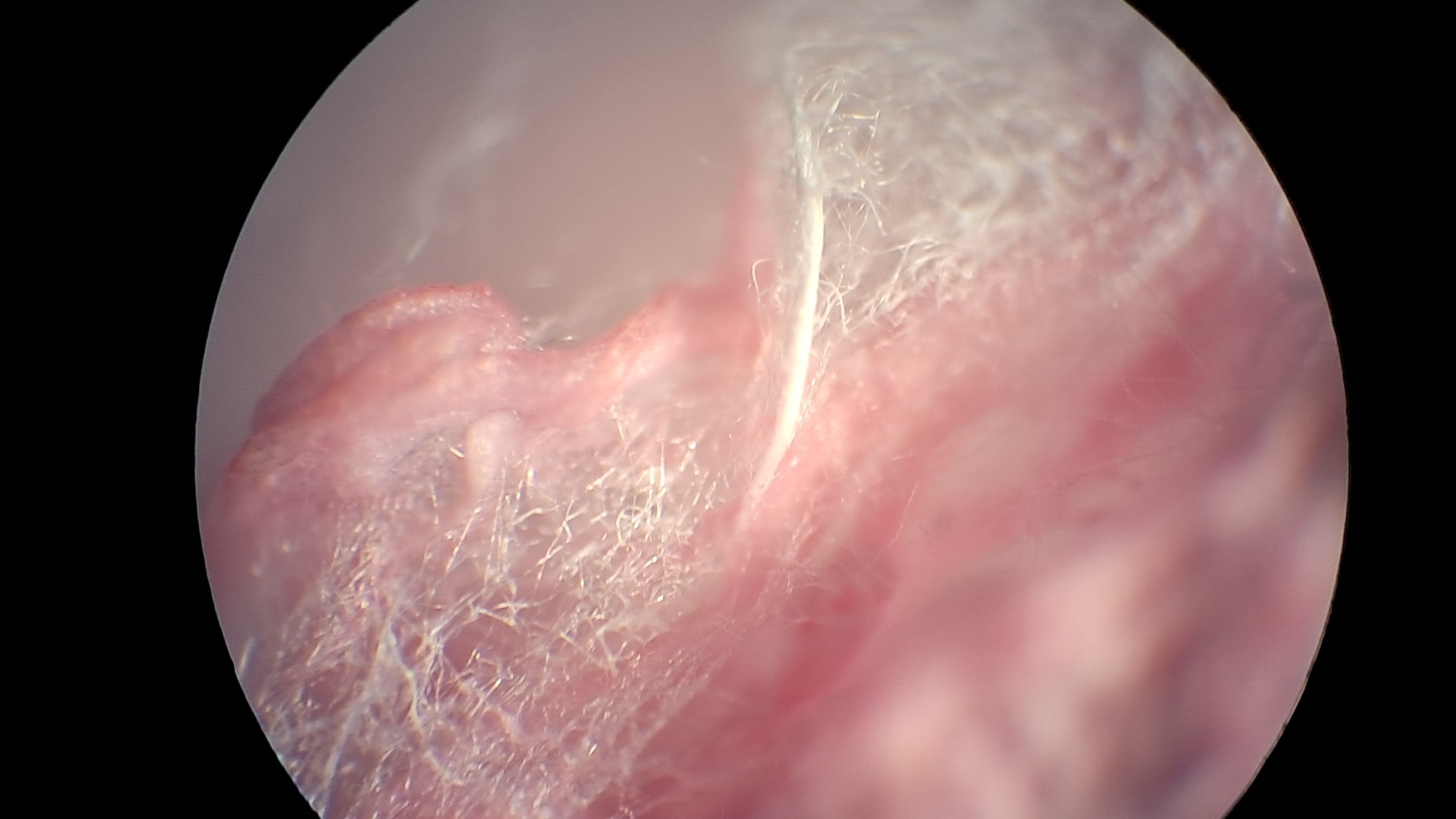
Электроспиннинг: поливинилхлорид и полистирол - Часть 33 I weighed 4.985 g of THF and 1.075 g of PVC insulation, cut the insulation into pieces, and added them to the solvent. I stirred the mixture periodically. By the next day, a crimson, highly viscous liquid had formed in the bottle. An attempt at electrospinning yielded negative results. Not even an aerosol formed - the electrostatic forces could not overcome the surface tension of such a viscous solution. I could have added a small amount of THF to reduce the viscosity. However, I decided to increase the chances of a positive result by mixing this solution with a 28.9% polystyrene solution in DMF. Since a solution of polystyrene in DMF or THF readily forms fibers during electrospinning, I assumed that its mixture with a PVC solution would also produce fibers. I mixed 1.250 g of the PVC solution with 1.225 g of the polystyrene solution. The mixture was highly viscous, so I added 0.440 g of DMF and stirred the components thoroughly. I began electrospinning. This time, the electrostatic forces ejected streams from the tip of the needle. The collector became coated with a layer of fibrous material. However, the material was unevenly colored: pink in some areas and white in others. Microscopic examination revealed that the material consisted of white fibers and irregularly shaped red particles. It was logical to assume that these particles were PVC. When I told my physicist colleague about the results of the experiment, he replied, "It didn't even completely dissolve!" He meant that the PVC had not fully dissolved in THF; part of the polymer had merely swollen and broken down into small particles, forming a suspension. These particles were what had landed on the collector. The bright crimson dye contained in the insulation had prevented me from noticing the presence of solid polymer particles in the solution. At the same time, I prepared a solution of the same polymer in DMF. The concentrations of the two solutions were approximately equal. When I carefully examined the PVC solution in DMF, I discovered small green PVC particles - again, some of the polymer had not dissolved but had swollen and formed a suspension. The insulation used to prepare this solution was predominantly dull green, which did not interfere with visual observation. After these results, I stopped working with PVC, although my colleague later brought me some film, claiming it was pure PVC. He also gave me an adhesive that was a ready-made PVC solution. |

Dissolving Polyvinyl Chloride in THF |

|

|

|

|

|

|

|

|

Electrospinning |

1.250 g of the PVC solution were mixed with 1.225 g of the polystyrene solution and 0.440 g of DMF |

|

|

|

Electrospinning |

|

|

|

|

|

|

|

|

|

|

|

|

|
|
|
|
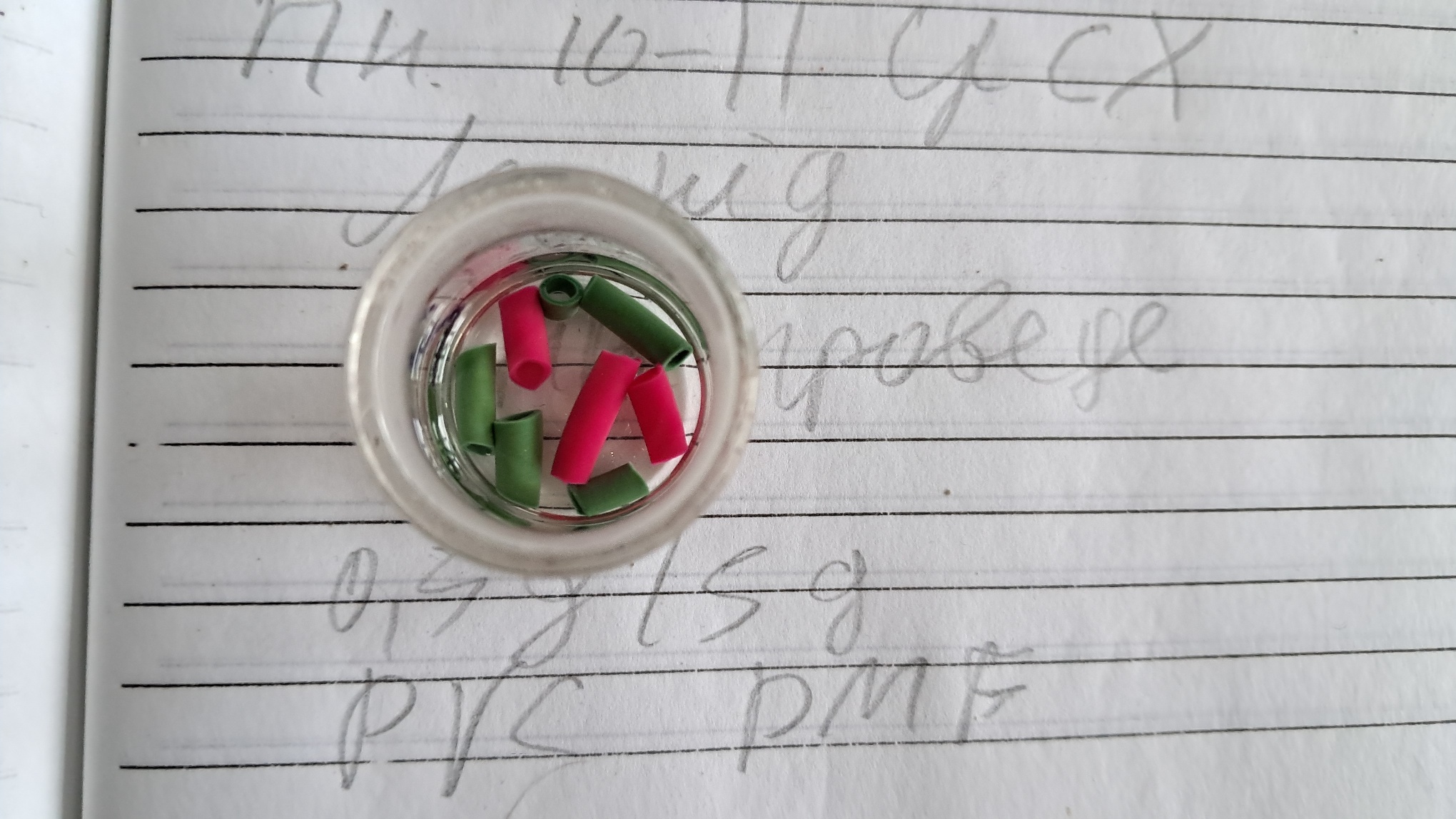
Dissolving Polyvinyl Chloride in DMF |

|

|

|

|

|

|
The unheated laboratory |

When I opened the cabinet, the acetic acid was still liquid - acetic acid is known for its ability to supercool without crystallizing. However, as soon as I carefully lifted the bottle, the liquid began to crystallize, turning into a white mass resembling ice.
|

The laboratory windows (interior side) |

|

|

|

A small portion of the acetic acid remained liquid |

The other laboratory |

Chitosan, acetic acid, and methyl acetate |

Chitosan |

Water |

Acetic acid |

Chitosan in: acetic acid/water, water, and methyl acetate/acetic acid |

|

|

Electrospinning |

|
|

Over the following days, the laboratory temperature dropped to 10°C, and the acetic acid in the bottle solidified completely. |