Chemistry and Chemists № 1 2026
Journal of Chemists-Enthusiasts
| Content | Chemistry experiments - video | Physics experiments - video | Home Page - Chemistry and Chemists |
|
Chemistry and Chemists № 1 2026 Journal of Chemists-Enthusiasts |
Electrospinning - pt.7, 8 Chemist |
|
Having noticed a mistake in the text, allocate it and press Ctrl-Enter
Dissolving Expanded Polystyrene in Ethyl Acetate - Part 7
Electrospun polystyrene was successfully obtained, but the exact composition of the "Acetone+" solvent used was unknown. Therefore, it was advisable to replace this solvent with one of known composition. Ideally, a pure compound should be used as the solvent, although technical mixtures of known composition (for example, gasoline) are also acceptable.
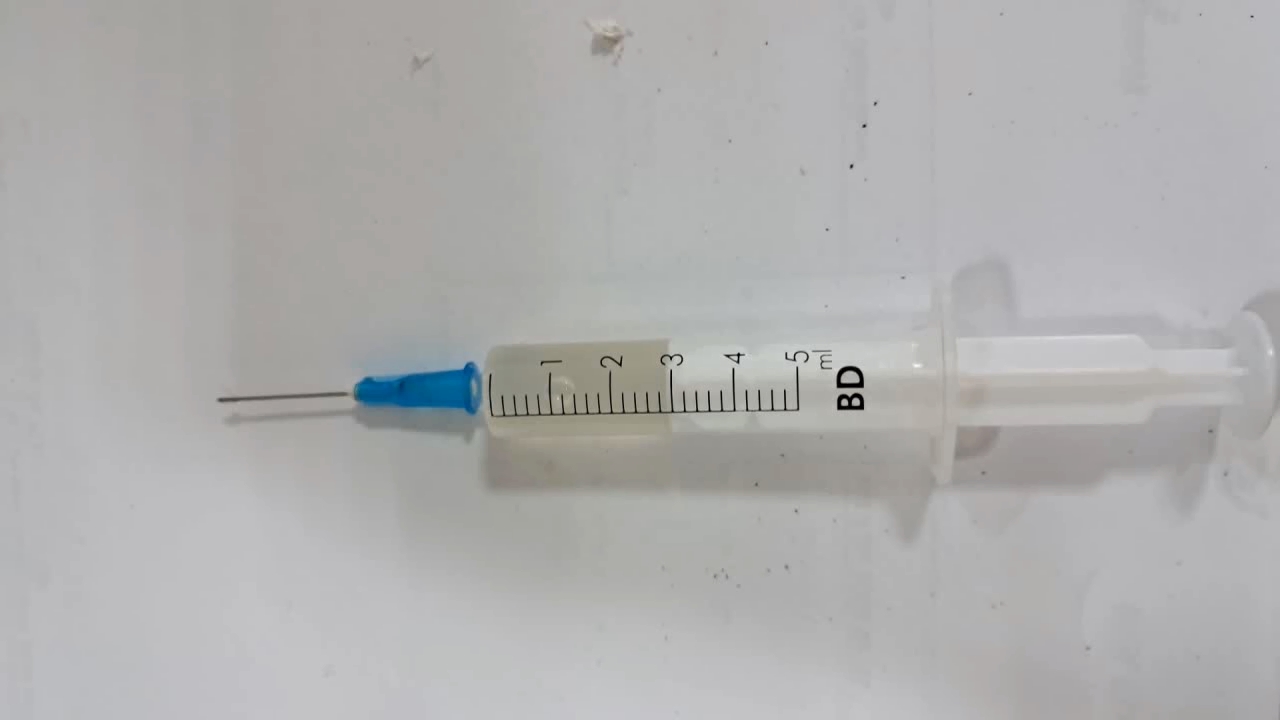
Растворение вспененного полистирола в этилацетате - Часть 7 I assumed that acetone was the best solvent for expanded polystyrene (polystyrene foam). However, as mentioned above, this substance is strictly restricted in our country. Colleagues were able to lend me a small amount of acetone, but it was sufficient only for trial experiments. Therefore, it made sense to look for an alternative solvent that was not subject to such restrictions. The laboratory had ethyl acetate and extraction gasoline. I consulted boiling point data. Ethyl acetate boils at 77.1°C. Gasoline of this type, however, boils in the range of 80-120°C, which is unacceptable - the upper limit is too high. Such a solvent would evaporate poorly during electrospinning (at least in our setup). Using gasoline could therefore lead to a repetition of the negative results observed in the experiments with PVDF solutions in dimethylformamide. For comparison, the boiling point of acetone is 56°C. I therefore chose ethyl acetate. I weighed 10.120 g of the solvent into a weighing bottle. I did not know the solubility of polystyrene in ethyl acetate and was not even certain that expanded polystyrene would dissolve in it at all. Therefore, I began by weighing 1.000 g of expanded polystyrene. I placed the first piece of expanded polystyrene into the weighing bottle containing ethyl acetate. Gas was released, and the polystyrene quickly dissolved, forming a colorless, cloudy solution. Subsequent pieces of polystyrene also dissolved rapidly. It seemed as though large pieces of expanded polystyrene were disappearing before my eyes. A small number of undissolved polystyrene particles, a few millimeters in size, remained in the liquid, but these dissolved later as the solution stood. I then weighed out another 1.020 g of expanded polystyrene and added it to the ethyl acetate with stirring. The new portions dissolved more slowly, but they eventually disappeared as well. As a result, a cloudy, colorless solution of polystyrene in ethyl acetate was obtained. Would electrospinning work with this solution? |

Dissolving Expanded Polystyrene in Ethyl Acetate |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
Having noticed a mistake in the text, allocate it and press Ctrl-Enter
Electrospinning: Solution of Polystyrene in Ethyl Acetate - Part 8
Having only limited experience with electrospinning, I could not confidently predict how a solution of polystyrene in ethyl acetate would behave. The solvent has a higher boiling point than the initial boiling point of the "Acetone+" solvent I had used previously. Therefore, problems with solvent evaporation during fiber formation were expected. As a result, fibers that had not yet dried could coalesce on the collector electrode into a continuous, semi-liquid mass.
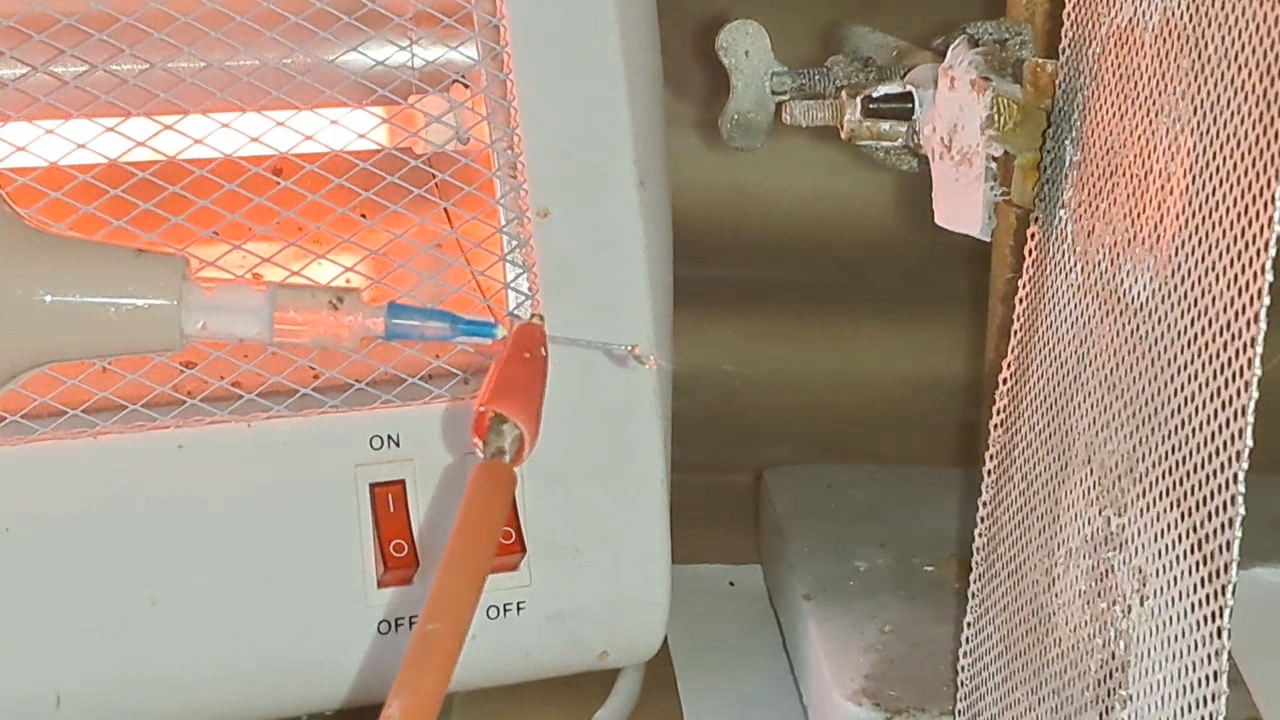
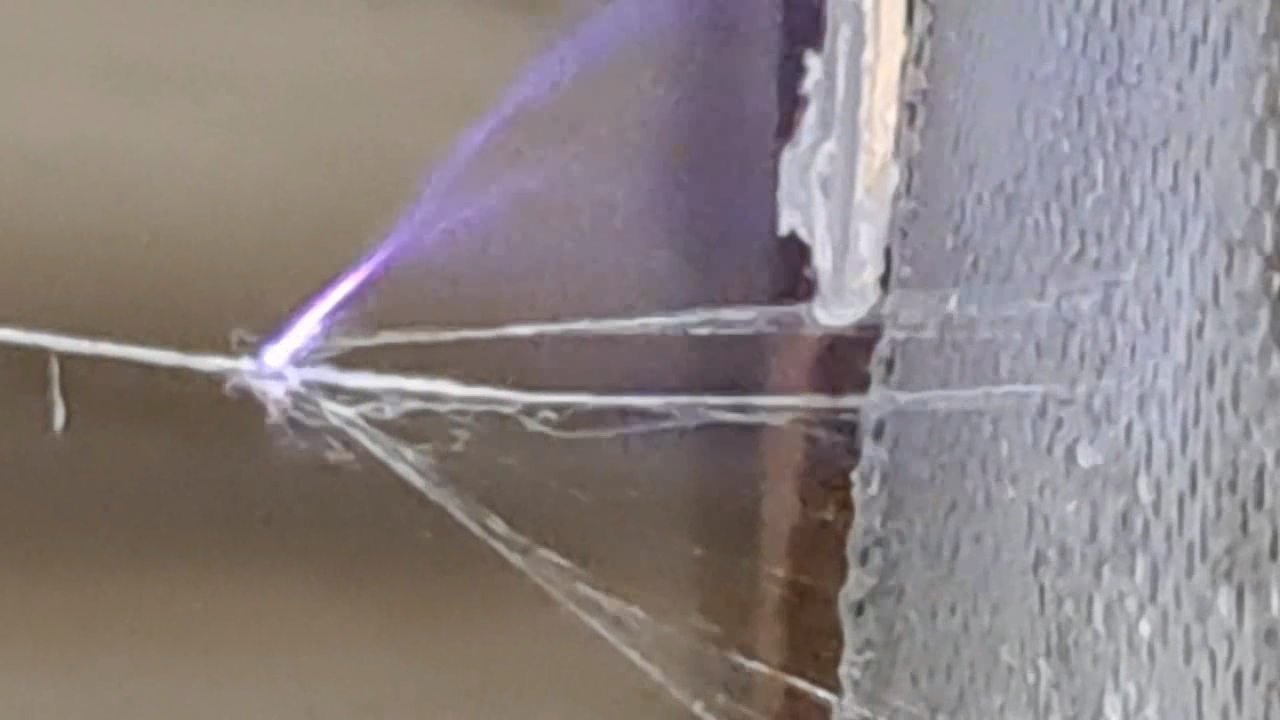
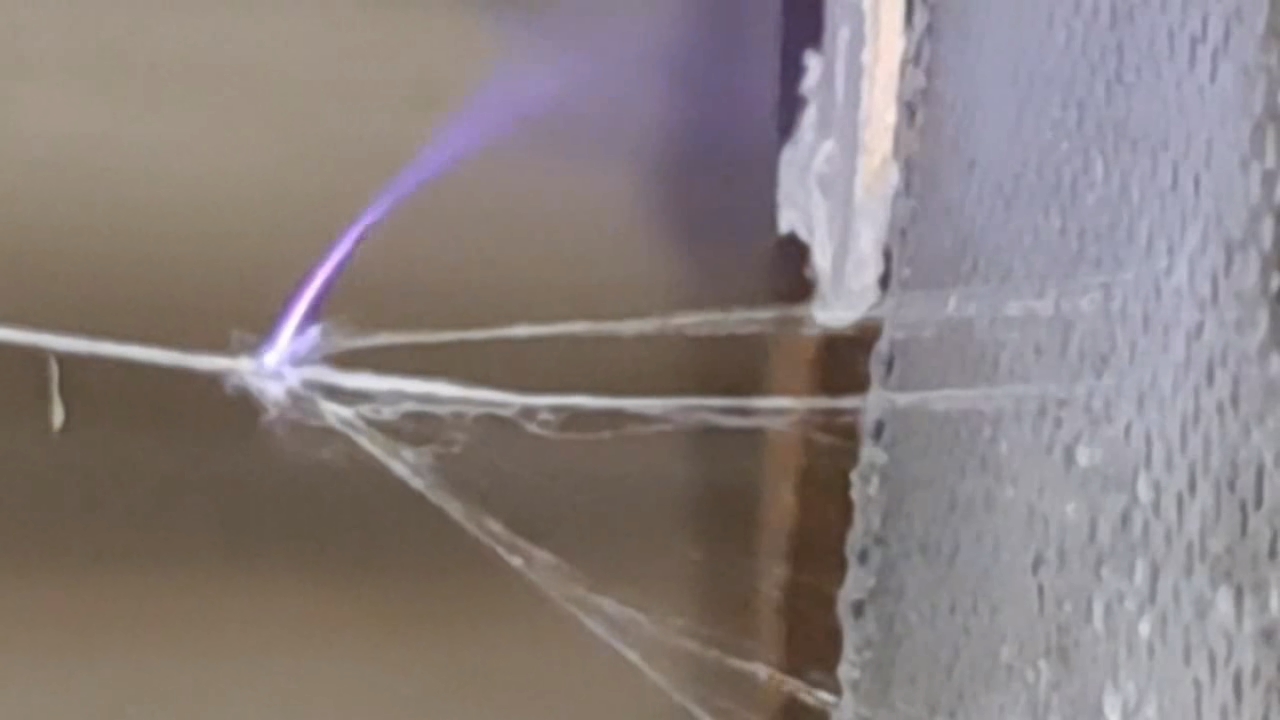
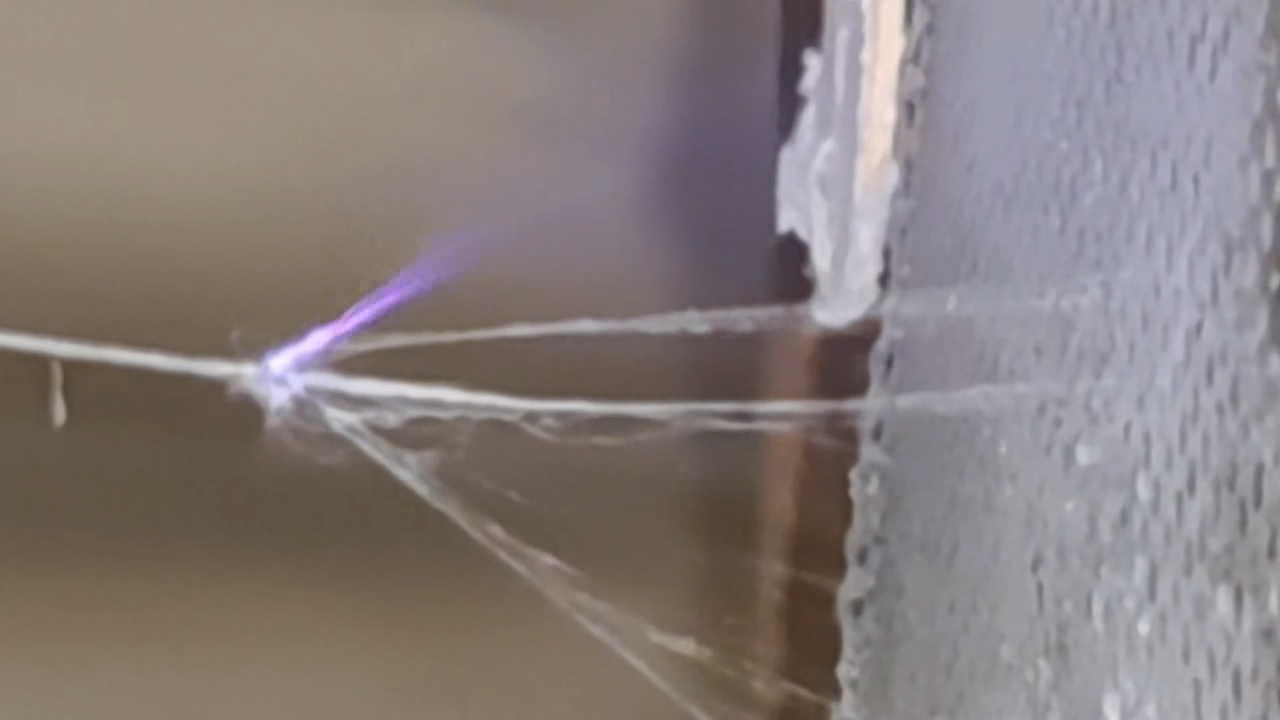
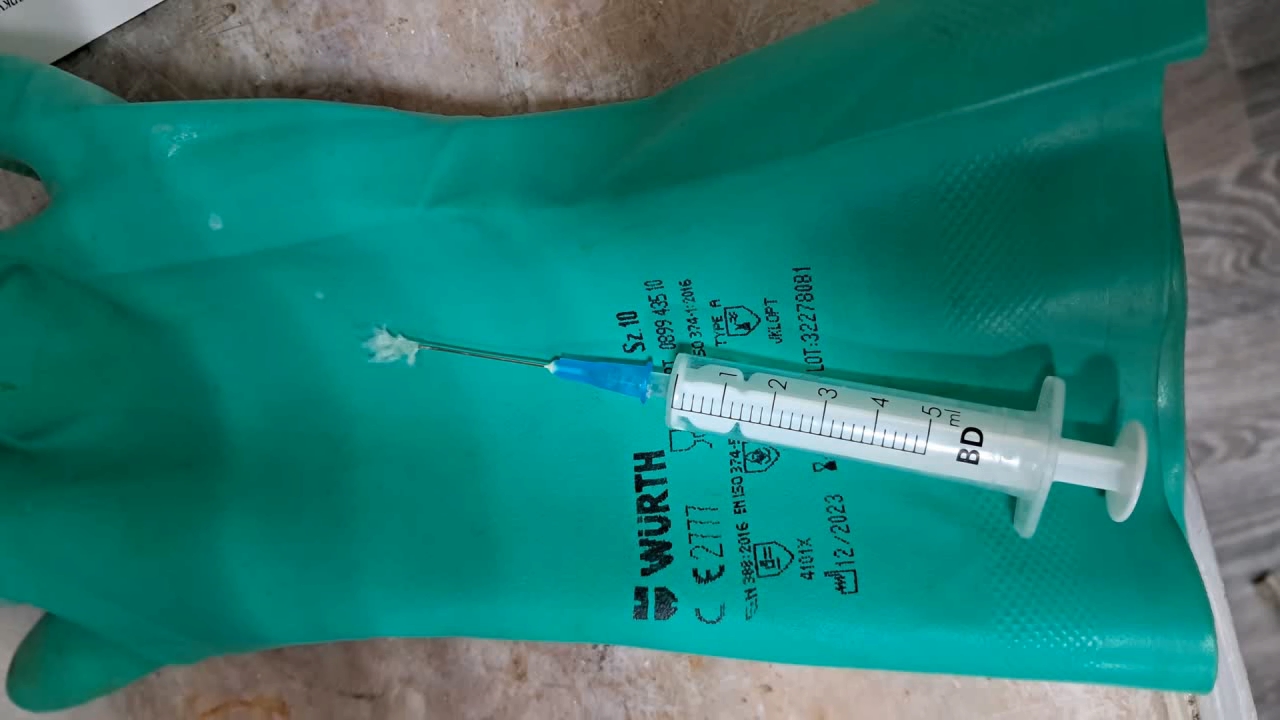
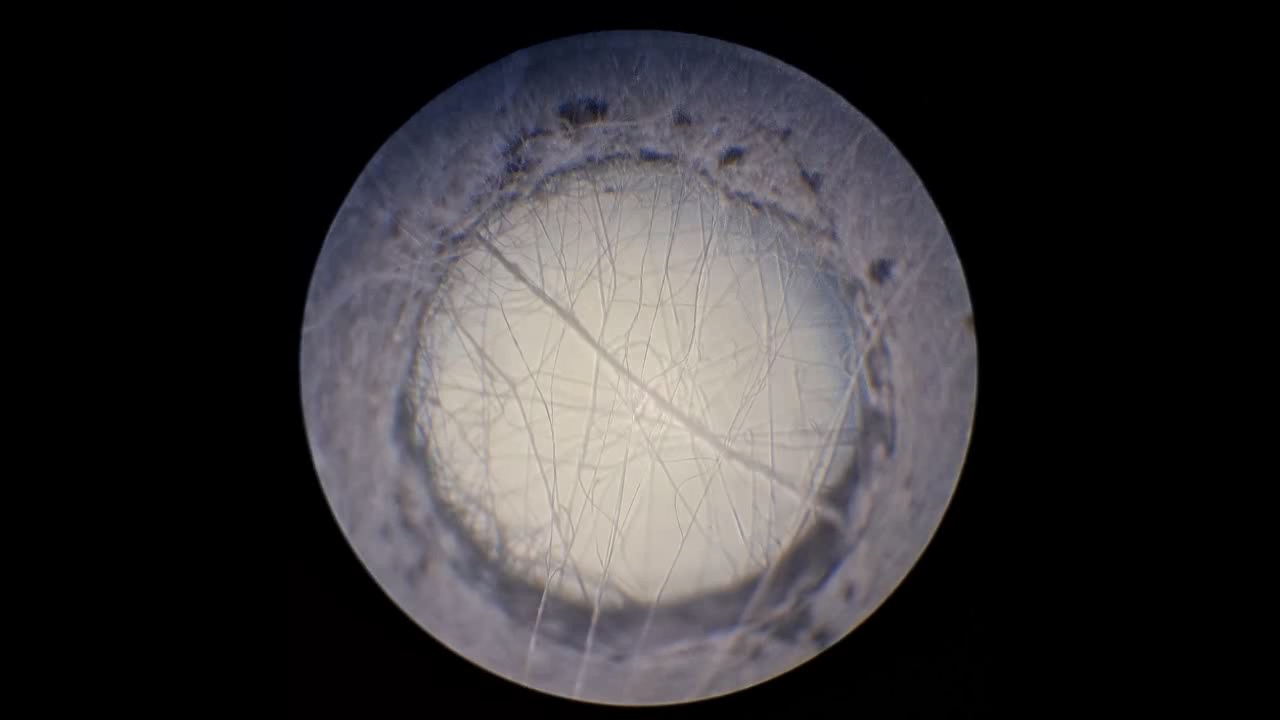
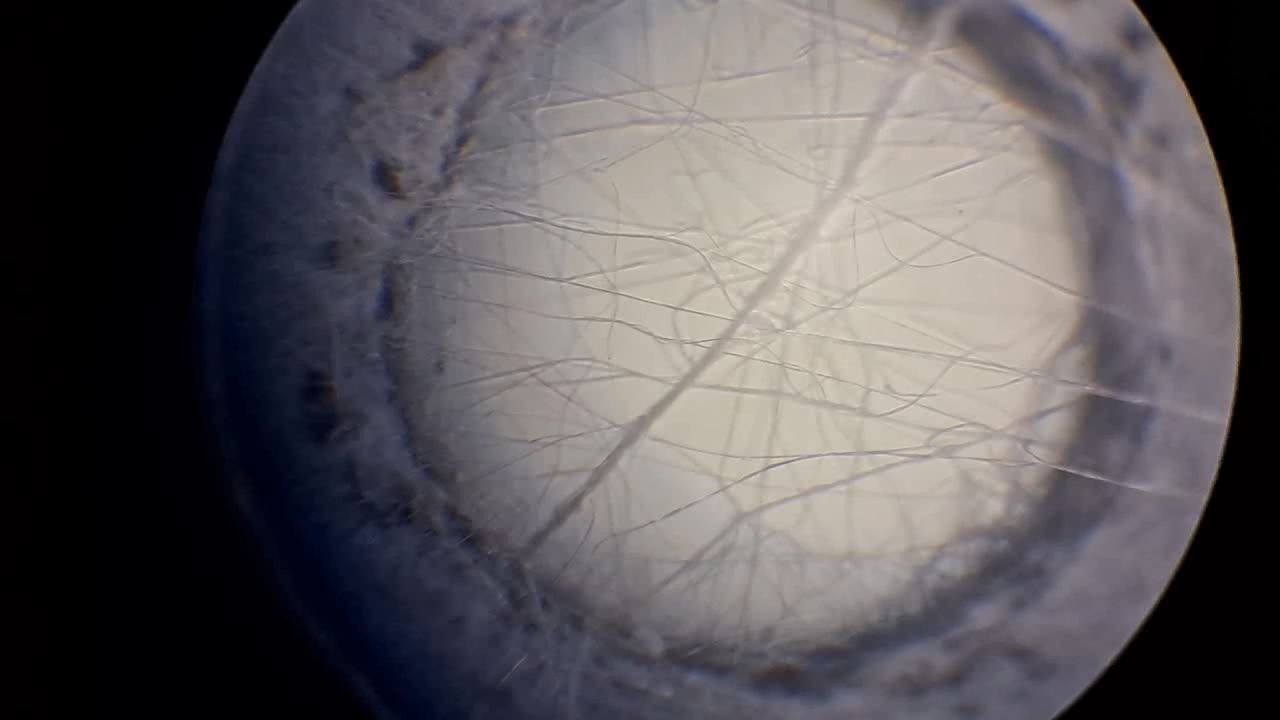
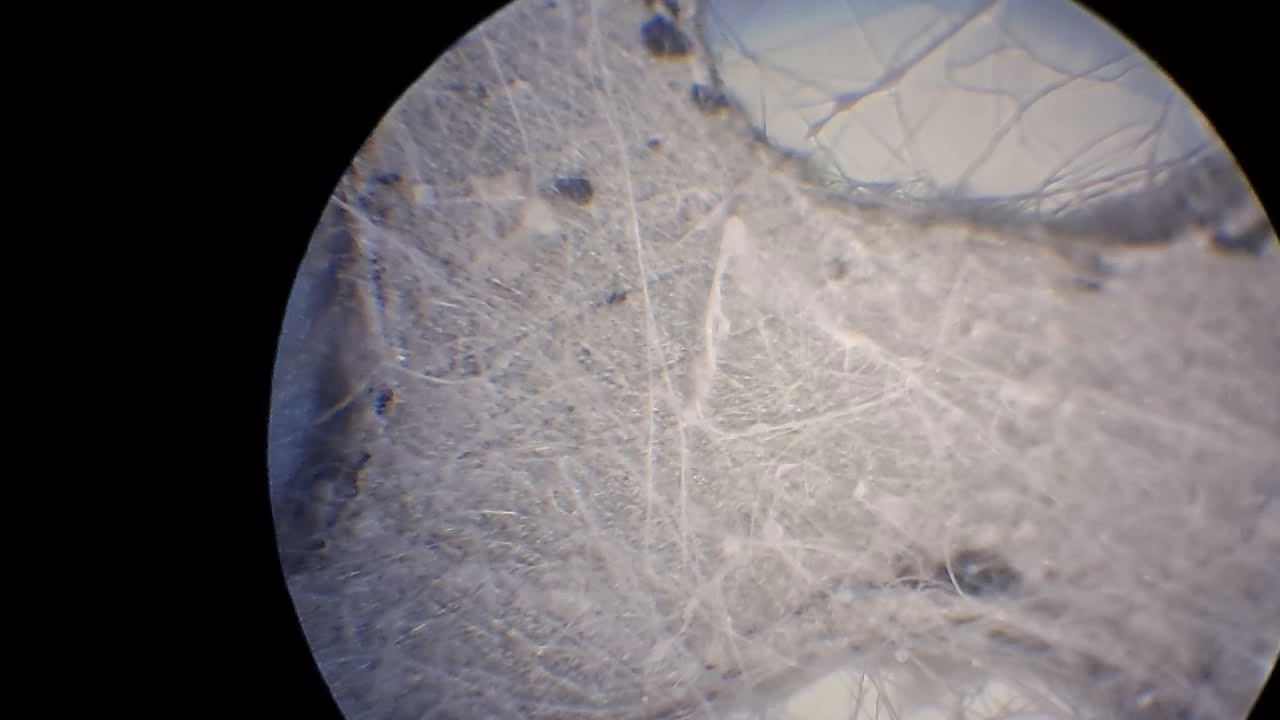
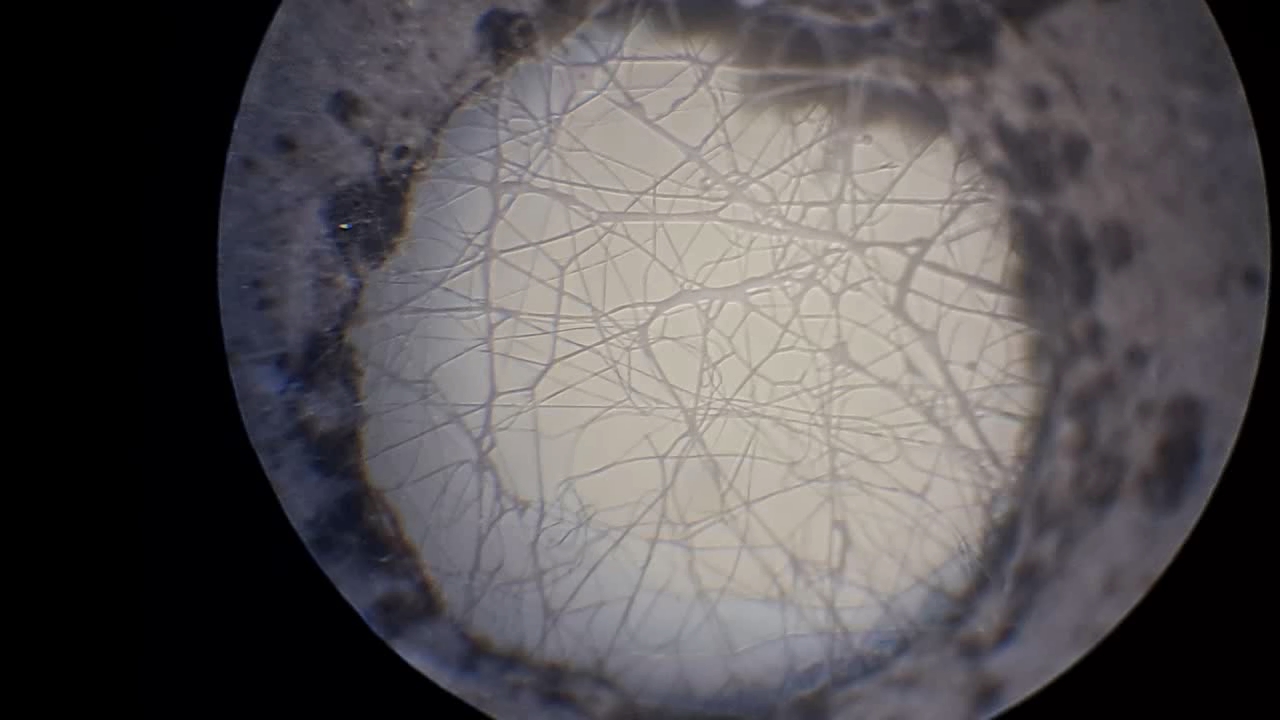
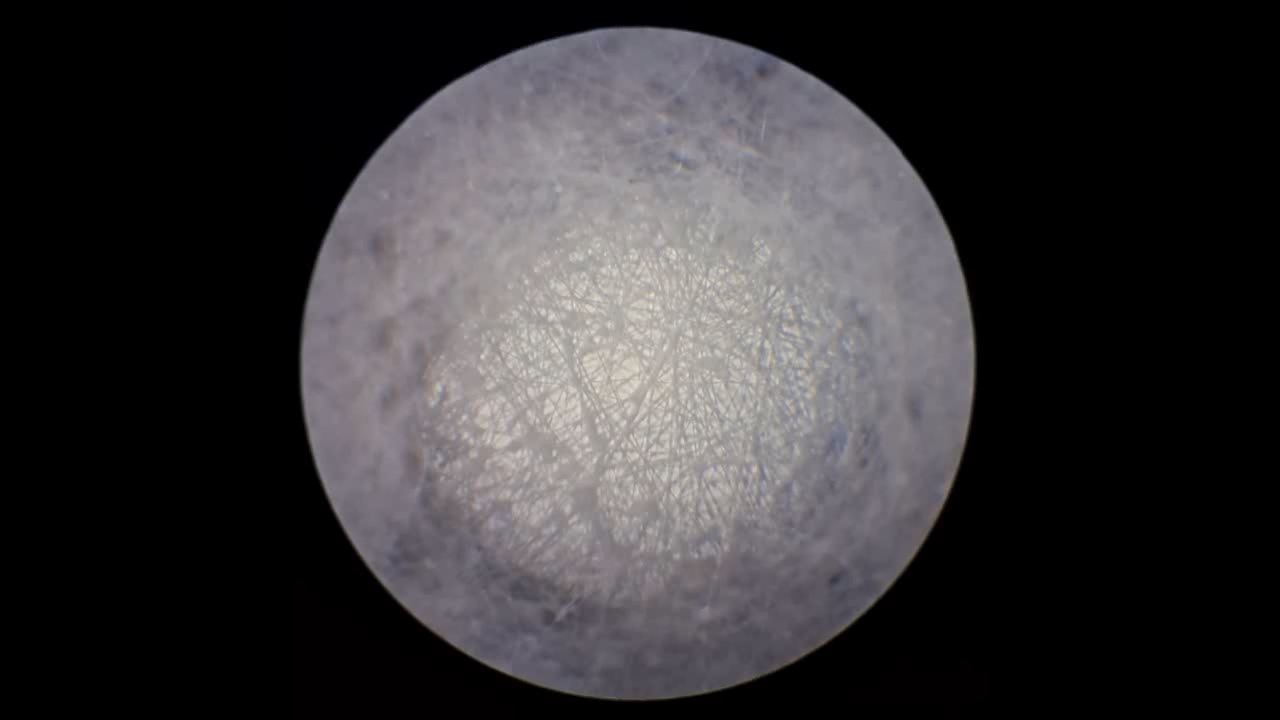
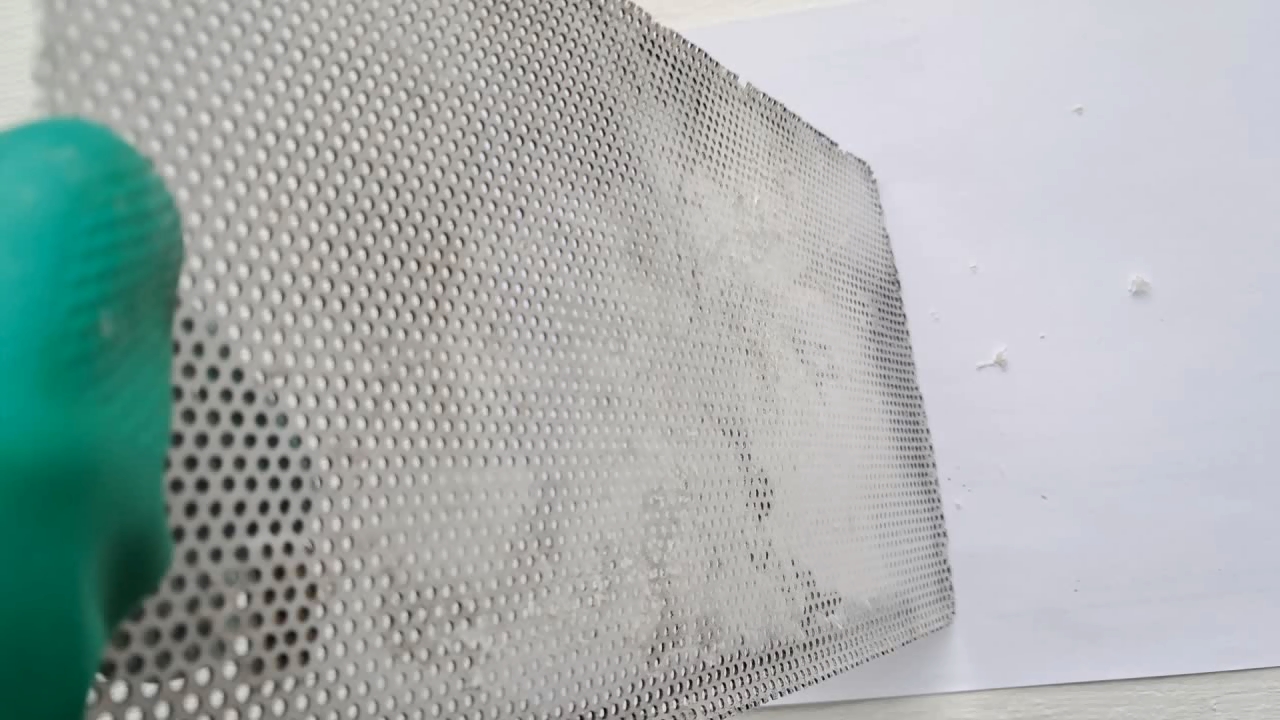
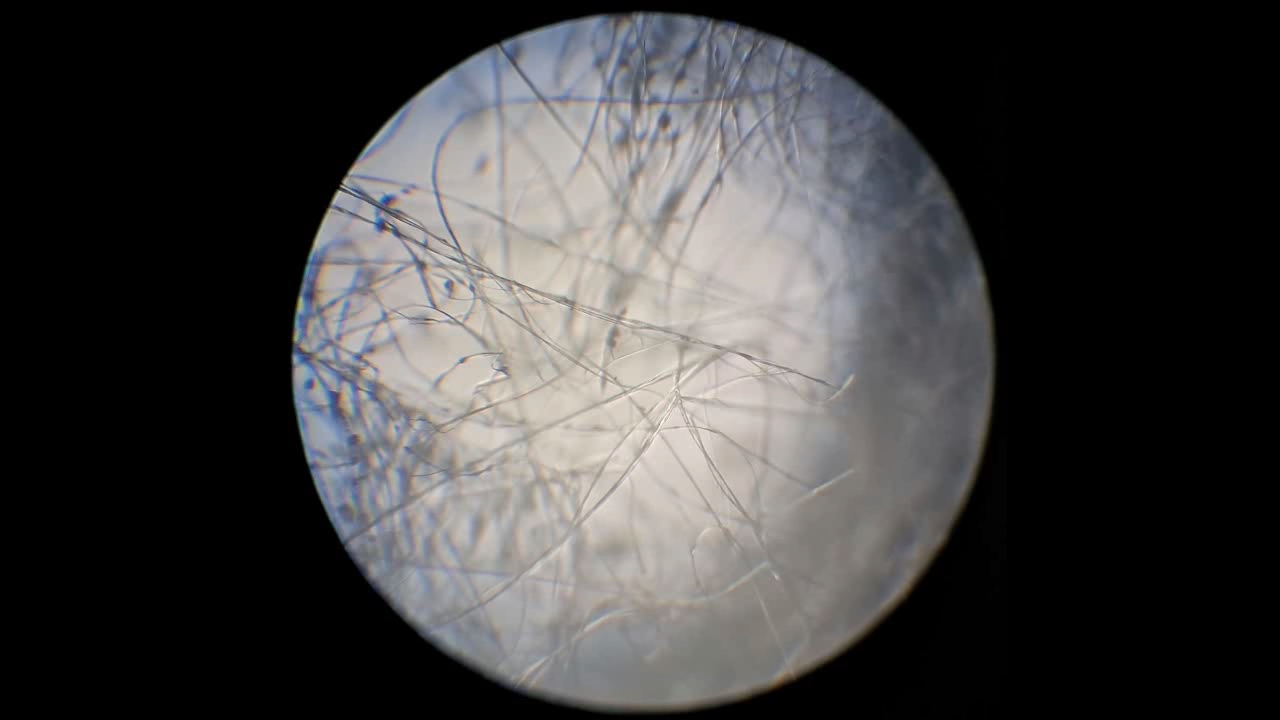
Электроспиннинг: раствор полистирола в этилацетате - Часть 8 To facilitate microscopic examination of the electrospun polystyrene structure, I initially used a metal mesh as the collector. This electrode could be removed from the setup at any time, allowing the resulting coating to be examined under a microscope. The holes in the mesh made it possible to observe the fibers in transmitted light. After examination, the electrode could be returned to the setup, and electrospinning could be continued. On the other hand, once the process was complete, separating the resulting material from the mesh proved much more difficult than from a smooth metal plate, because the holes in the mesh hindered removal. Therefore, after several preliminary experiments, I replaced the metal mesh with aluminum foil and later with a solid metal sheet. To promote complete evaporation of ethyl acetate, I placed the heater too close to the needle and syringe, forgetting to be cautious. I have already described the outcome: part of the setup overheated, the plastic softened, and the syringe and high-voltage electrode flew off. Fortunately, the high-voltage electrode did not strike me, even though I was standing nearby. I had to abandon the use of the heater altogether. Only several weeks later did I learn how to use it without endangering either the setup or myself. Another minor accident occurred when I placed the needle too close to the collector mesh. Once again, a corona discharge appeared, followed by a loud spark discharge. In the video recording, the spark appears as a brief flash, although the discharge channel - a miniature "lightning bolt" between the electrodes - was clearly visible to the naked eye. Fortunately, the transformer survived unharmed. What did this experiment show? The good news was that the process closely resembled the earlier experiments performed using the acetone substitute as the solvent. Fibers emerged from a droplet of solution protruding from the tip of the needle. Some of these fibers formed dendrites (a "beard") that did not detach from the needle. Over time, a white coating of electrospun polystyrene accumulated on the collector. Unfortunately, the ethyl acetate solution of polystyrene "spat" much more intensely than the polystyrene solution in "Acetone+." Numerous wet spots appeared on the white coating. After the experiment, it became clear that in these regions the solution had glued the fibers together into a continuous film, partially dissolving them. This effect was caused not only by the change in solvent but also by the absence of a heater. Microscopic examination revealed that the polystyrene fibers obtained from the ethyl acetate solution were significantly thicker than those produced using "Acetone+." In both cases, however, the electrospun material resembled cotton wool. As in previous experiments, some fibers were attracted not to the collector but to surrounding surfaces: retort stands, the walls and floor of the fume hood, and even the experimenter's hands. I assumed that the collector electrode should be properly grounded and that perhaps the electrical connection between the computer monitor and the collector was insufficient. First, I connected the collector electrode to a water faucet using a wire. Using water pipes as a grounding circuit is unacceptable, but I decided to do this briefly to test my hypothesis. I turned on the setup - nothing changed. A few seconds later, I realized that the water pipes in the laboratory were plastic; steel pipes were a thing of the past. I then found a long wire and connected the collector to the laboratory grounding circuit, located at the opposite end of the room. When I fastened the other end of the wire to the grounding terminal, I received a painful electric shock. It turned out that someone had connected a live wire to this grounding circuit - most likely using it in place of a neutral conductor. I put on rubber gloves, completed the connection, and performed the experiment. No significant change was observed after grounding the collector: some of the polystyrene fibers continued to attract to foreign surfaces. I conducted the experiment described above about a month ago. Since then, I have worked with several other solvents. Today, I returned to experiment with ethyl acetate, changing the flow direction from horizontal to vertical (top to bottom) and using a heater. This new ethyl acetate experiments are not yet complete, as frequent power outages are slowing progress. For now, I will describe my experiments with alternative solvents. |

Electrospinning: Solution of Polystyrene in Ethyl Acetate |
|

|
|

|

|

|

|

|

|

|
|
|
|

|

|

|
|

|
|
|
|
|
|

|
|

|

|

|

|

|

|

|

|

|
|

|