Chemistry and Chemists № 1 2026
Journal of Chemists-Enthusiasts
| Content | Chemistry experiments - video | Physics experiments - video | Home Page - Chemistry and Chemists |
|
Chemistry and Chemists № 1 2026 Journal of Chemists-Enthusiasts |
Electrospinning - pt.12, 13 Chemist |
|
Having noticed a mistake in the text, allocate it and press Ctrl-Enter
Dissolving Expanded Polystyrene in Methylene Chloride - Part 12
The next possible solvents for polystyrene were methylene chloride (boiling point ≈40°C) and tetrahydrofuran (boiling point ≈66°C). The colleague initially suggested using chloroform, dichloroethane, or methylene chloride, but I wanted to avoid toxic organochlorine solvents whenever possible. For this reason, I initially refrained from using organochlorines to dissolve expanded polystyrene and instead worked with the "Acetone+" solvent, ethyl acetate, methyl acetate, and acetone.
Растворение пенополистирола в хлористом метилене - Часть 12 The first two solvents dissolved polystyrene well, but their use led to incomplete evaporation of the solution during electrospinning, especially in the case of ethyl acetate. Since dichloromethane has a low boiling point, its use in electrospinning offered hope of overcoming the problem of insufficient solvent evaporation during the process. I weighed 13.720 g of methylene chloride and 3.435 g of expanded polystyrene. I began adding pieces of the polymer to the solvent. The expanded polystyrene dissolved rapidly, releasing gas bubbles. Dissolution proceeded faster than in the case of ethyl acetate or the "Acetone+" solvent. The process looked remarkable: a small volume of liquid quickly absorbed a large volume of solid material, although the dissolution rate gradually slowed. As with ethyl acetate, after most of the expanded polystyrene had dissolved, small white lumps remained in the liquid; these dissolved over time with continued stirring. The result was a slightly cloudy 20.0% solution of polystyrene in methylene chloride. |

Dissolving Expanded Polystyrene in Methylene Chloride |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
Having noticed a mistake in the text, allocate it and press Ctrl-Enter
Electrospinning: Solution of Polystyrene in Methylene Chloride - Part 13
The boiling point of dichloromethane is so low (about 40°C) that it is not much higher than normal human body temperature. Therefore, I expected the 20% polystyrene solution to evaporate without difficulty during electrospinning.
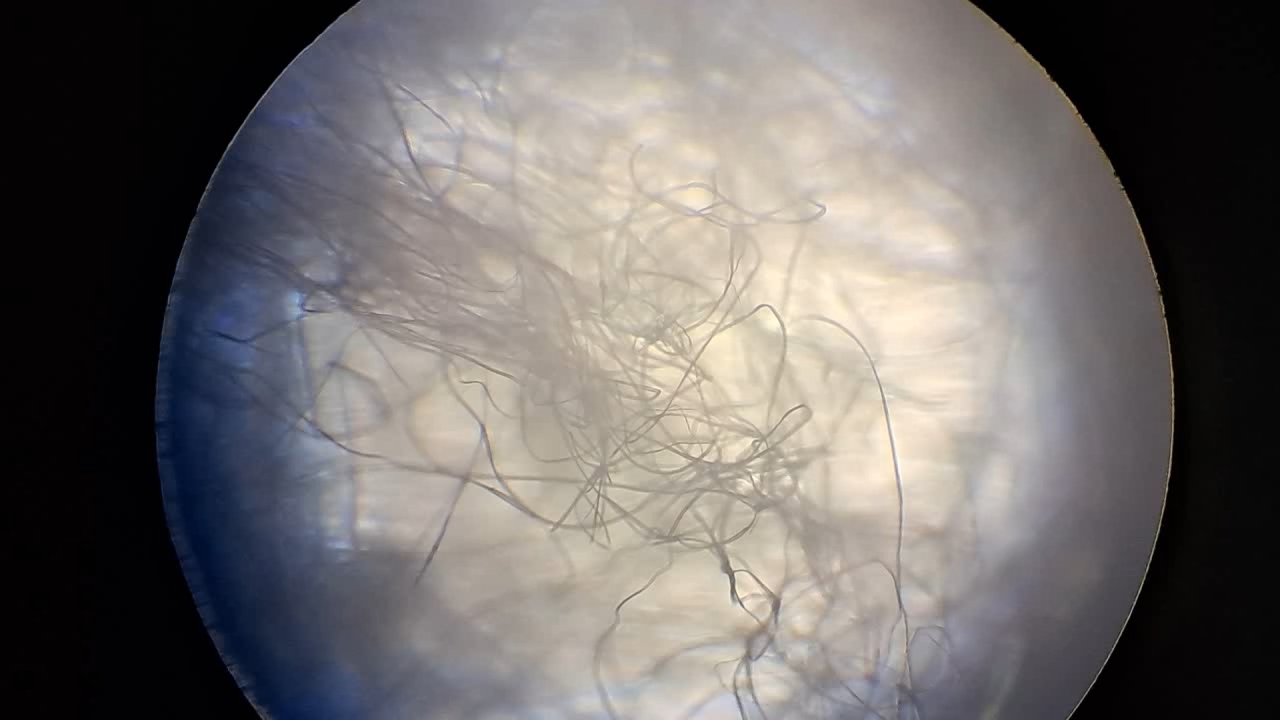
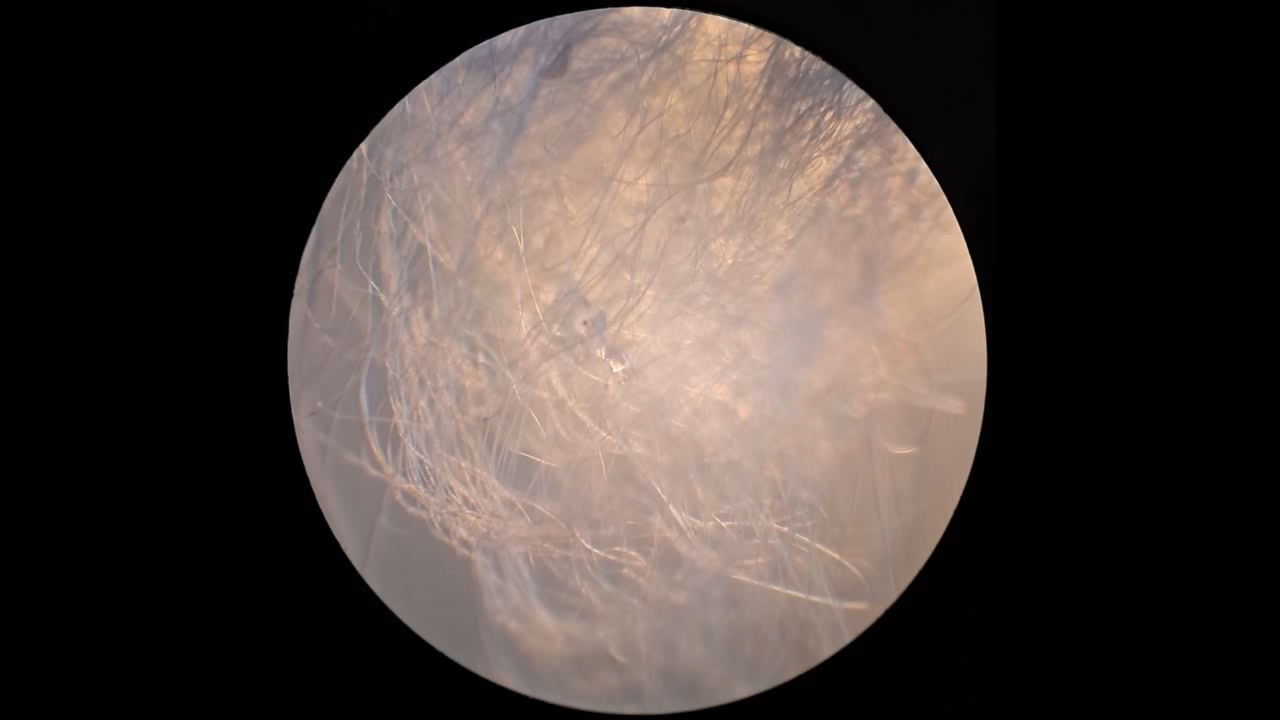
Электроспиннинг: раствор полистирола в метиленхлориде - Часть 13 I drew the solution into a syringe and began the process. The good news was that the polystyrene solution in dichloromethane began to form fibers, just as the polystyrene solutions in ethyl acetate and the "Acetone+" solvent had. The fibers settled on the collector, forming a white coating. However, my joy was premature. Along with the fibers, gel-like dendrites also formed, extending and branching without detaching from the needle. Taylor cones, from which the fibers originated, appeared directly on these dendrites. As the dendrites grew toward the collector, the distance between the points where the fibers formed and the collector decreased significantly. This led to the familiar problem of incomplete solvent evaporation, which had previously occurred with ethyl acetate and, to a lesser extent, with the "Acetone+" solvent. In addition, a large droplet often formed at the tip of the needle and continued to grow as more solution arrived. The electrostatic field was insufficient to effectively draw fibers from this droplet, because the surface tension of the solution was too high. I was frustrated. Could it be that dichloromethane is not an appropriate solvent for electrospinning? I stopped the process and examined the surface of the collector electrode under a microscope. The fibers were clearly visible, but in some areas there were signs of partial dissolution of fibers that had already been deposited on the collector. The presence of "beads" on the fibers was also noticeable. I diluted the solution with methylene chloride to a polystyrene concentration of 13.9% and resumed electrospinning. Fibers formed and were then attracted to the collector and to other surrounding surfaces, forming a "web." The process was still not entirely smooth. Dendrites continued to form, but they were now "dry," resembling a "beard," and no longer served as channels through which the solution approached the collector. As a result, the collector became coated with a white, cotton-like material. No wet spots were observed. The reverse side of the collector was also partially covered with polystyrene fibers. I examined the resulting material under a microscope. Numerous fibers without "beads" were visible. The fiber diameter was larger than that obtained with the "Acetone+" solvent and was roughly comparable to the diameter of fibers produced from a polystyrene solution in ethyl acetate. Unlike other solvents I have worked with, the odor of dichloromethane was barely noticeable. Given the toxicity of this compound, the lack of a clearly perceptible odor is a negative factor. I later learned that the solvent has a sweet, ether-like odor, but people quickly become accustomed to it and do not perceive methylene chloride at low concentrations. The odor becomes noticeable only when its concentration exceeds safe limits. I conducted electrospinning in a closed fume hood, but switched the hood on only occasionally, as a strong, uncontrolled airflow interfered with the process. In later experiments, I observed fibers detaching from the needle but failing to adhere to the collector or any other surface, as they were carried away by even a weak air current. Having confirmed that methylene chloride could be used for electrospinning polystyrene, I began experiments with the less toxic tetrahydrofuran. |

Electrospinning: Solution of Polystyrene in Methylene Chloride |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|

|

|
|

|