Chemistry and Chemists № 1 2026
Journal of Chemists-Enthusiasts
| Content | Chemistry experiments - video | Physics experiments - video | Home Page - Chemistry and Chemists |
|
Chemistry and Chemists № 1 2026 Journal of Chemists-Enthusiasts |
Electrospinning - pt.16, 17 Chemist |
|
Having noticed a mistake in the text, allocate it and press Ctrl-Enter
Dissolving Polystyrene in Surrogate Acetone - Part 16
In previous experiments, I varied the solvents and polymer concentrations but used the same polystyrene sample throughout: to prepare working solutions for electrospinning, I dissolved expanded polystyrene in various organic solvents. Of course, different polystyrene samples can differ in their degree of polymerization and, consequently, in their physical properties - particularly in their ability to dissolve in organic solvents. I accidentally discovered that one expanded polystyrene sample dissolved in acetone, while another only swelled. The first sample also appeared to "melt" upon contact with hexane, whereas the second remained unchanged when immersed in hexane. There is no doubt that differences in the properties of different polystyrene samples can affect the electrospinning process.
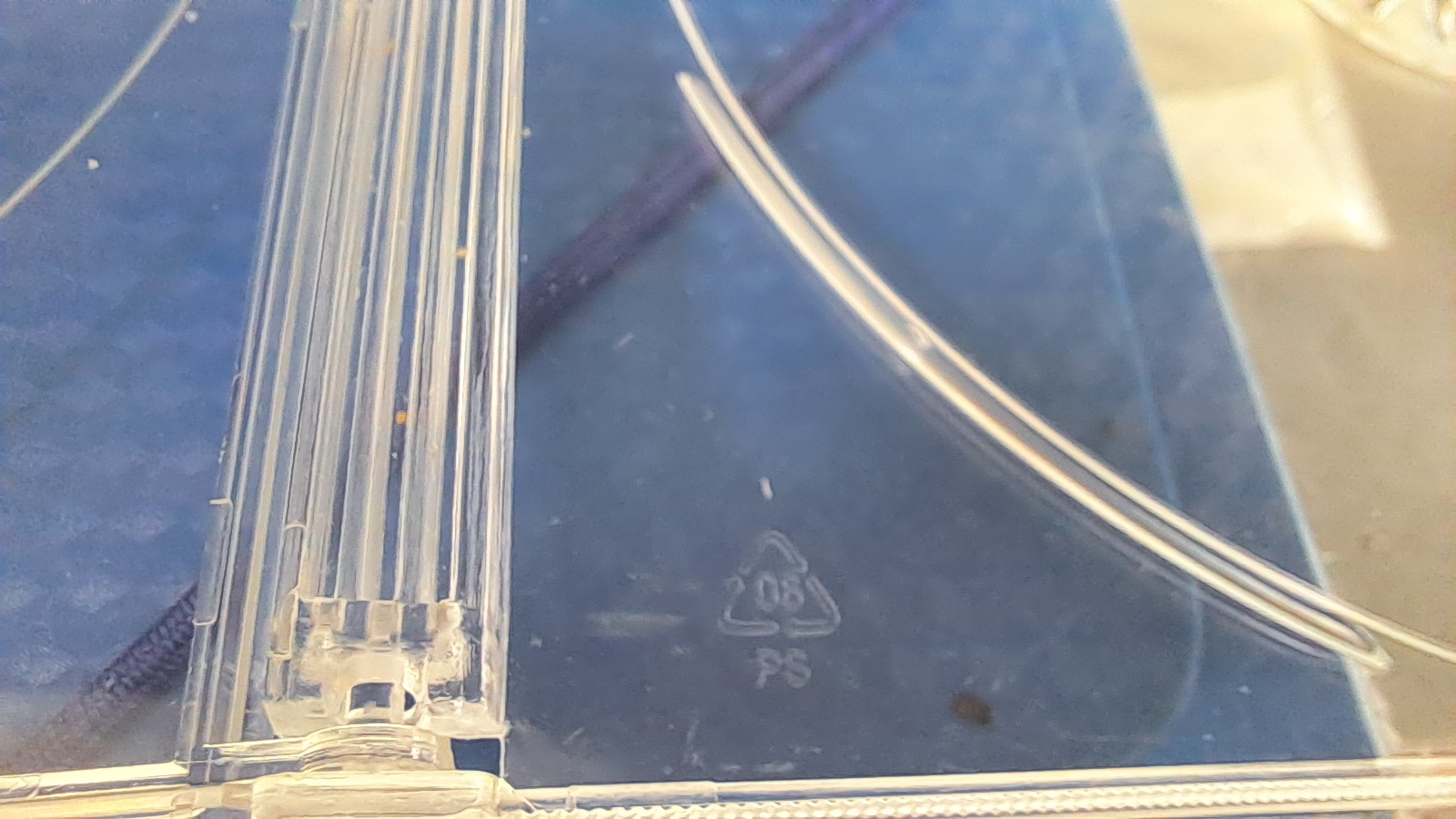
Растворение полистирола в суррогатном ацетоне - Часть 16 Therefore, it became necessary to prepare a working solution using a different polystyrene source. The chemist colleague recommended using disposable plastic tableware as a source of the polymer, claiming that it was primarily made of polystyrene (and more rarely of polyvinyl chloride). The laboratory contained disposable plates, cups, spoons, and forks left behind by my predecessors. In fact, the abundance of kitchenware in the lab gave the impression that people came there to eat and drink rather than to work - but that is another story. I clearly saw the "PP" marking on the cups (polypropylene). The plates were marked, but the polymer type was not specified. The disposable forks and spoons were unmarked and resembled polystyrene. When ignited, the material burned with a smoky yellow flame and emitted an odor similar to that of burning expanded polystyrene. Nevertheless, I decided not to risk making a mistake and chose to look for another source of polystyrene. I remembered that I had several DVD boxes. An instructive story is associated with these boxes. Many years ago, the same colleague was asked by his research supervisor to develop a low-cost material for bulletproof vests. The war had just begun. The work was voluntary: he received no salary for it and even used his own reagents and glassware. The colleague knew that polymethyl methacrylate (PMMA) could stop bullets while being significantly lighter than steel. He therefore decided to dissolve PMMA in carbon tetrachloride, soak sandpaper in the solution, and then press dense layers from it. He used CD and DVD boxes as a source of PMMA. For reasons unknown to me, he was absolutely convinced that these boxes were made of polymethyl methacrylate. He poured about two liters of carbon tetrachloride into a flask and dissolved a large number of pieces of these boxes in it. I took his word that the boxes were made of PMMA, but such a frivolous waste of a hard-to-find solvent irritated me. Meanwhile, he heated pieces of these boxes in a Wurtz flask to depolymerize PMMA and obtain methyl methacrylate. As a result, some liquid was distilled, although a significant portion of the material charred and turned into a black mass. The colleague claimed that, according to the procedure, Rose's alloy should be added to the polymer to ensure more uniform heating. I do not know what science fiction book he read this in - of course, I did not believe it. By that time, I had already noticed that he often unconsciously distorted facts he had read or heard, sometimes beyond recognition. I doubted the use of Rose's alloy, but I was confident that the boxes were made of polymethyl methacrylate. I photographed and videotaped the dissolution and depolymerization and most likely even published it in our journal, stating that the polymer was PMMA. The body armor plates my colleague produced failed testing. The customer was angry and demanded that he continue the work. The colleague refused: since he had received no payment (and had not expected any), he was under no obligation to continue. The customer himself was not a volunteer - he had hoped to profit from supplying body armor to the army. My colleague lost interest in the project and abandoned it entirely. The flask containing the remaining solution (more than a liter) stood under a fume hood for many years, until wall tiles fell off and shattered the flask along with several bottles of tritiated water. The monomer obtained by depolymerization likely evaporated from the loosely sealed receiver. About ten years later, I was watching a chemistry video on YouTube in which an amateur chemist described the construction of an electrolysis cell. I was not particularly interested in the topic itself, but I watched the video to practice English. Suddenly, my interest was piqued. The author used a sulfonated polystyrene membrane in the cell. To prepare it, he cut a square from an identical DVD box and treated the polymer with concentrated sulfuric acid. This meant that the boxes were not made of PMMA, but of polystyrene (PS). At the time, I did not want to upset my colleague, so I did not tell him anything. However, I now needed a source of polystyrene. He immediately noticed that I was using the same type of transparent DVD box that he had used years earlier, still believing it to be polymethyl methacrylate. My box was clearly marked "PS" with the number 6 inside a triangle - polystyrene. He was upset and, to reassure himself, claimed that disc boxes had once been made from a copolymer of PMMA and PS. He also stated that transparent, colorless polystyrene is rare. He is very good at inventing explanations on the spot. I did not believe him, but I did not argue, so as not to upset him further. I weighed 1.535 g of polystyrene (the box pieces) and placed it in a bottle with 10.005 g of the "Acetone+" solvent, choosing the least expensive option. Naturally, this did not produce the same spectacular video as dissolving expanded polystyrene. The pieces of the box gradually swelled and dissolved over the course of about an hour. To speed up the process, I periodically stirred the contents with a wooden stick. A slightly cloudy solution with a polystyrene concentration of 13.3% formed; I deliberately reduced the polymer concentration. |

Dissolving Polystyrene in Surrogate Acetone |
|

|

|

|

|

|

|

|

|

|

|

|
|
Having noticed a mistake in the text, allocate it and press Ctrl-Enter
Electrospinning: Solution of Polystyrene in Surrogate Acetone - Part 17
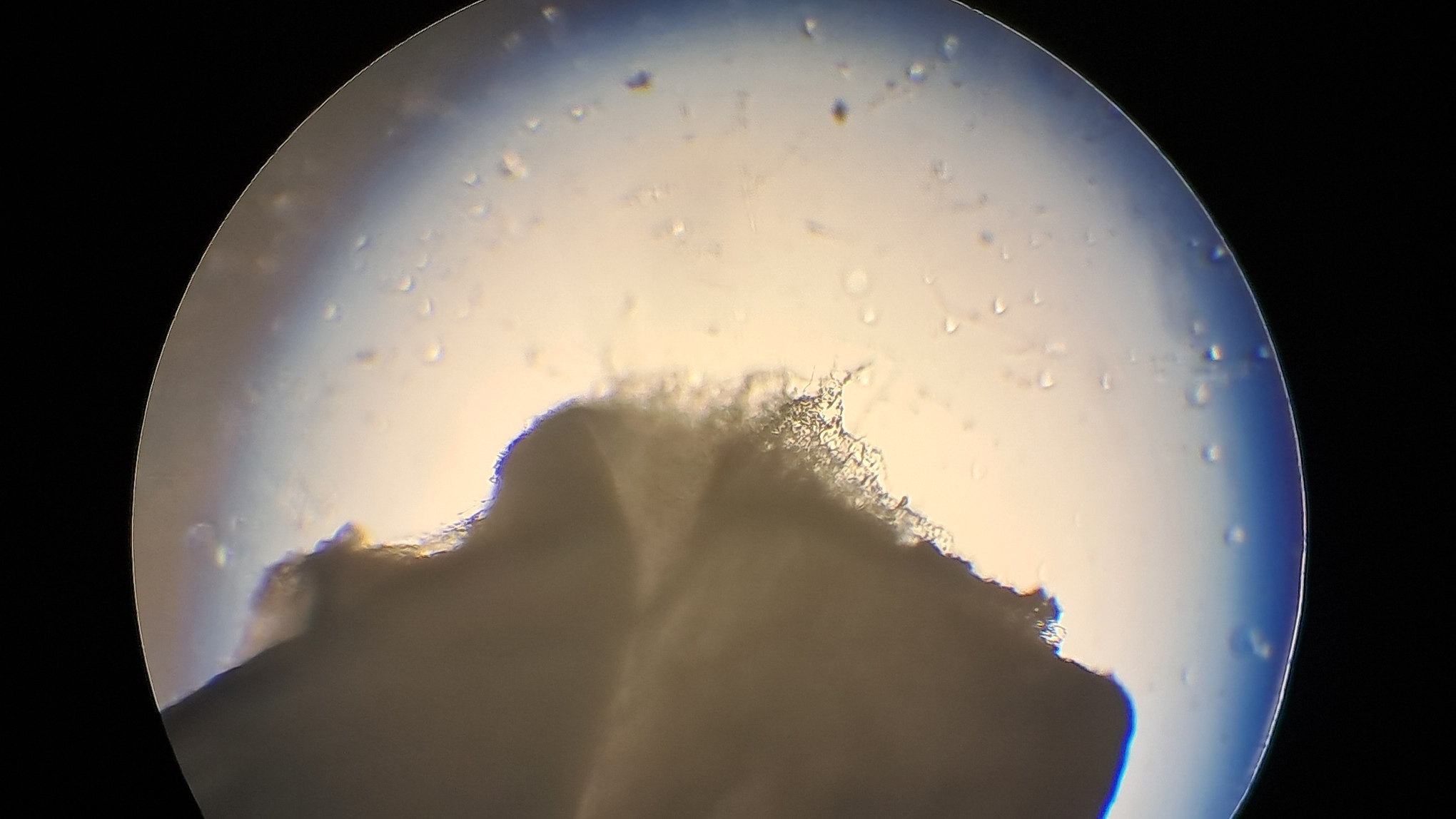
To my relief, electrospinning the new polystyrene sample in "Acetone+" (13.3%) proceeded in much the same way as electrospinning the previous polystyrene sample. Polymer fibers "shot" out of the needle, a white "beard" formed at the needle tip, and the collector was coated with a white layer of fibrous polystyrene. To prevent fibers from depositing on the reverse side of the collector, I covered it with a plastic sheet. This caused the fibers to deposit on the plastic instead, and the fact that the plastic sheet was a dielectric did not prevent this undesirable effect.
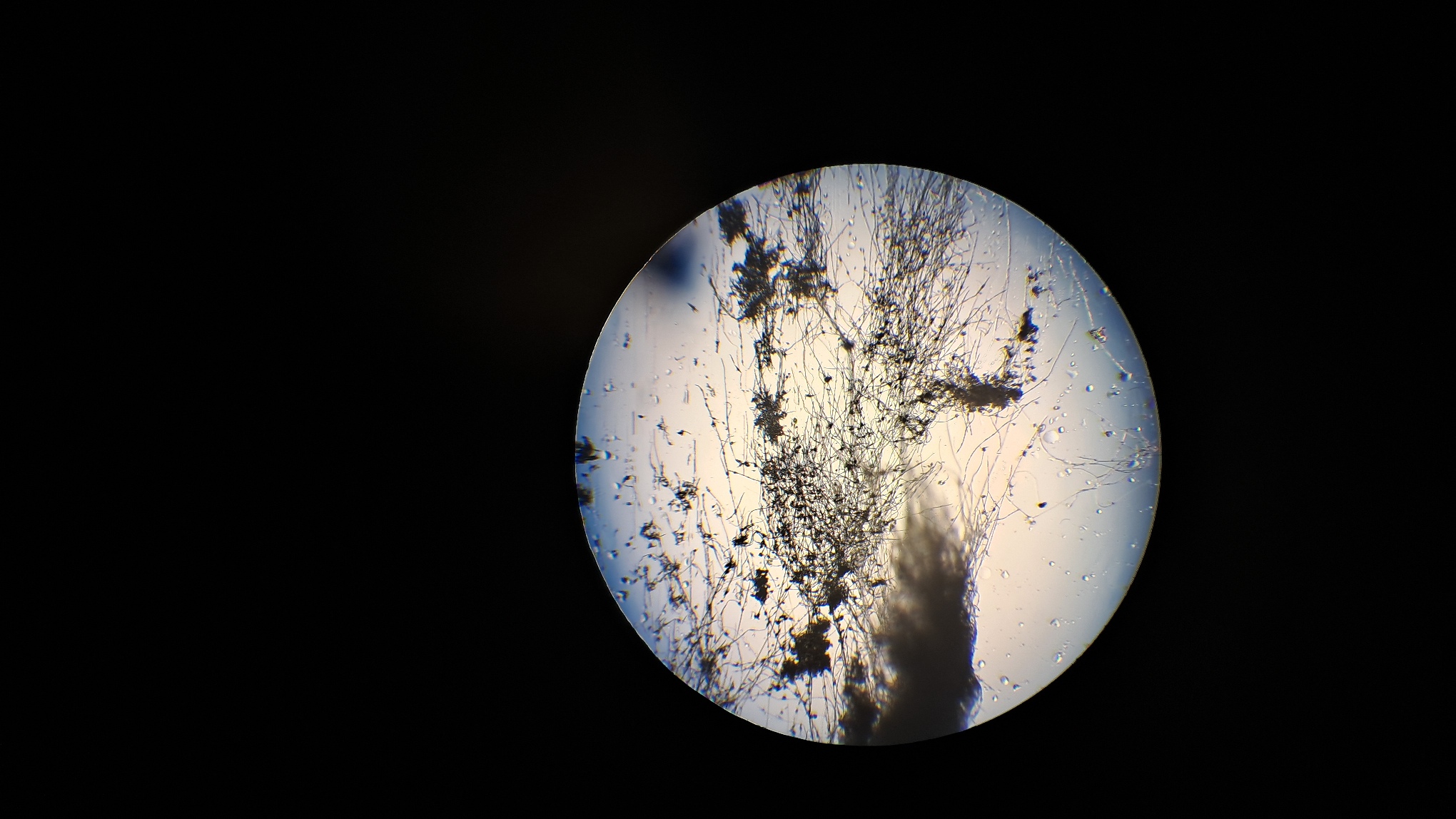
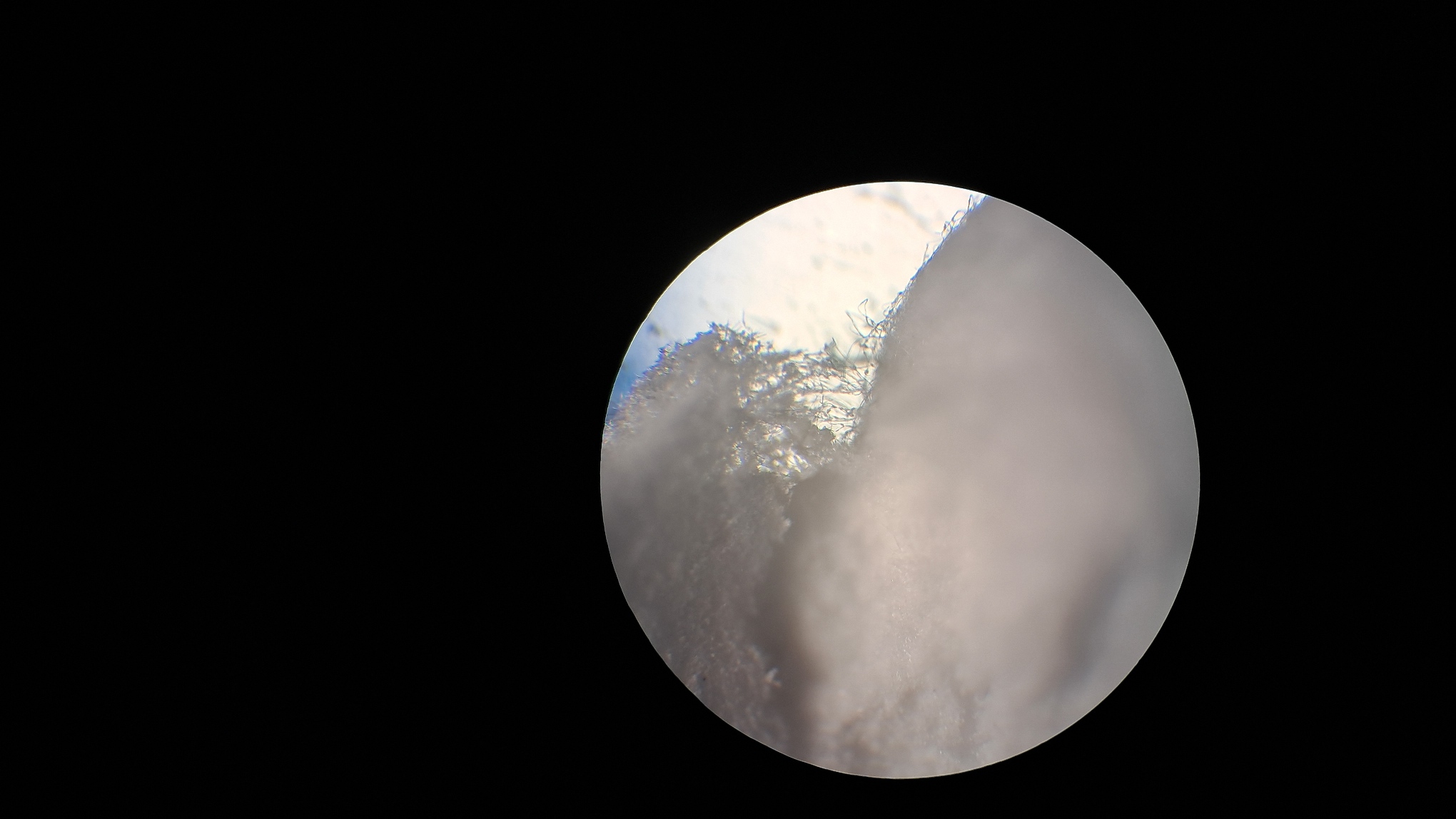
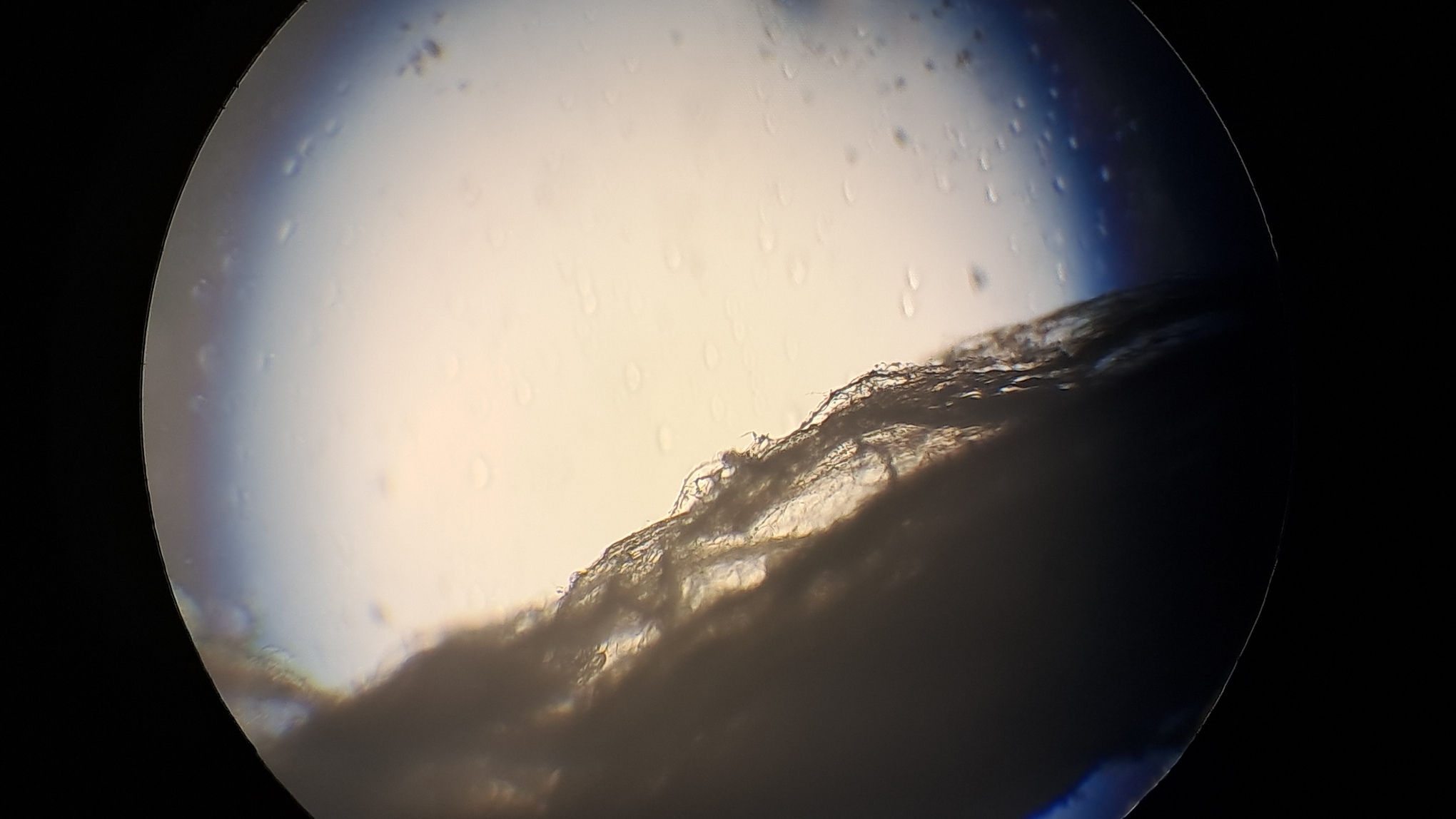
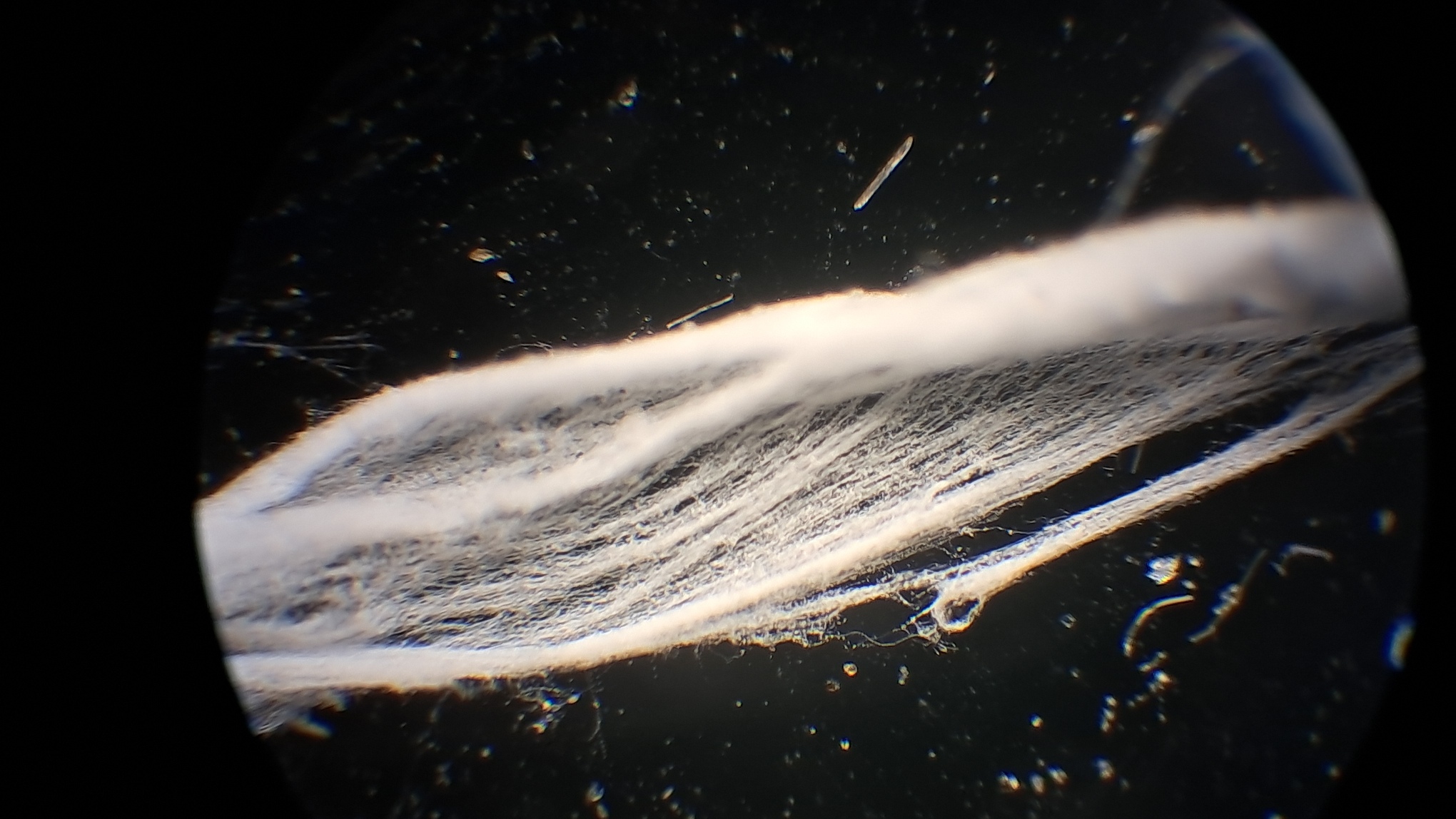
Электроспиннинг: раствор полистирола в суррогатном ацетоне - Часть 17 Microscopic examination of the resulting material clearly revealed the presence of fibers. Their diameter roughly corresponded to that of fibers obtained from a solution of expanded polystyrene in the same solvent. To investigate the effect of polystyrene concentration, I diluted the original solution with "Acetone+" to a concentration of 6.8% and subjected it to electrospinning. A white coating formed on the collector, but the process did not go smoothly. During electrospinning, the solution frequently "spat": droplets of solution reached the collector and dissolved the already deposited coating. Microscopic examination showed that the coating consisted of fibers, but their diameter was significantly smaller than that of fibers obtained from solutions with a higher polystyrene concentration in the same solvent. Visually, the fiber diameter was roughly comparable to that of fibers obtained from tetrahydrofuran, although in the case of tetrahydrofuran the polystyrene concentration was approximately twice as high. At this point, I decided to stop experimenting with polystyrene and move on to other polymers. |

Electrospinning: Solution of Polystyrene (13.3%) in Surrogate Acetone |

|

|

|

|

|

|

|

|

|
|

|

|
|

|

Electrospinning: Solution of Polystyrene (6.8%) in Surrogate Acetone |

|

|

|

|

|

|

|

|

|

|

|

|
|
|

|
|

|

|

|

|

|

|

|

|

|

|
|
Комментарии
К1
Конечно же, коробочки для дисков и тому подобное никогда не делают из ПММА. Это вообще не литьевой полимер - т.е. для отливки из него изделия его понадобилось бы нагреть до температуры разложения. Он, как правило, делается в виде листов или очень простых заготовок. Для этого мономер с инициатором заливают между вертикальными листами силикатного стекла и нагревают. Дальше - механическая обработка и/или изгиб при нагревании.Существуют, конечно, разные акриловые сополимеры, в том числе литьевые. Из них коробочки не делают уже по экономическим соображениям. Полистирол дешевле во много раз и отлично льётся. И бронежилет из акриловых полимеров не сделать, они недостаточно прочные. Делают из лексана, это довольно дорогой поликарбонат. |