Chemistry and Chemists № 1 2026
Journal of Chemists-Enthusiasts
| Content | Chemistry experiments - video | Physics experiments - video | Home Page - Chemistry and Chemists |
|
Chemistry and Chemists № 1 2026 Journal of Chemists-Enthusiasts |
Electrospinning - pt.18, 19 Chemist |
|
Having noticed a mistake in the text, allocate it and press Ctrl-Enter
Dissolving Polymethyl Methacrylate (PMMA) in Methylene Chloride - Part 18
In response to my question, the chemist colleague recommended using organochlorine solvents - methylene chloride, chloroform, or dichloroethane - to dissolve polymethyl methacrylate (PMMA). I chose methylene chloride because it has the lowest boiling point. He also provided me with several PMMA samples. These included a plexiglass plate from his electrodialysis unit, several transparent rods, and a plastic Petri dish.
Растворение полиметилметакрилата (PMMA) в метиленхлориде - Часть 18 I did not believe the Petri dish was made of PMMA, recalling the story with the DVD boxes (see Part 16). It was tempting to use the transparent rods to prepare the solution, since the plate was thick and durable and difficult to break into pieces. However, I had doubts - the rods looked more like polystyrene than polymethyl methacrylate. To test this hypothesis, I placed one of the rods in a flame. The plastic melted and burned with a yellow, smoky flame, emitting an odor similar to burning polystyrene. From childhood, I remembered that burning PMMA smells completely different. In the literature, this odor is often described as "the smell of rotten vegetables." I had to go to another laboratory to break off pieces of the PMMA plate with a hammer. It was winter, and the room was unheated - the temperature was only a few degrees above freezing. When I entered the lab, I was horrified: it was cold and damp. How had I ever worked in this room before? I hammered off several pieces of the plate, which should have been sufficient for my experiments. I had no glass containers or bottles available, so I had to carry out the dissolution in a PET bottle, even though I knew this plastic might be unstable in methylene chloride. I placed 1.565 g of polymethyl methacrylate and 9.875 g of dichloromethane into the bottle and screwed the cap on tightly. The bottle began to turn white in places and wrinkle, and the PMMA started to swell. After some consideration, I added another 6.025 g of dichloromethane. The next day was Christmas Eve (called "Holy Evening" in our language), but during wartime, holidays are officially considered working days in our country. In practice, no one at the institute forces employees to come in on holidays - it is voluntary. I came because I wanted to continue the experiments. I did not arrive in the morning, but at 1:00 p.m., when the power supply was scheduled to resume. When I arrived, I discovered that the walls of the PET bottle had wrinkled significantly, and the polymethyl methacrylate and dichloromethane had formed a transparent gel that did not flow. I had hoped that a flowing solution would form, which I could use for electrospinning once the power was restored. I quickly emptied a glass weighing bottle that had previously contained a polystyrene solution and transferred the gel into it. I added 12.830 g of methylene chloride and stirred the contents. The gel dissolved slowly, so I added another 5.990 g of the solvent. Half an hour had passed since the power was supposed to return, but it still had not been supplied. I checked the provider's schedule and found that the restoration had been postponed until 4:00 p.m. Meanwhile, power had returned at my home, so I went there, intending to come back to the lab once electricity was restored. That evening, the weather became noticeably colder - temperatures of -10°C and below are rare here. When I returned, power had finally been restored at the institute. By that time, a viscous solution had formed in the weighing bottle containing the polymer and solvent. The viscosity was clearly too high for electrospinning, but the solution had already been diluted substantially - the PMMA concentration was only 4.3% (1.565 g PMMA in 34.720 g dichloromethane). I decided to draw the solution into a syringe, hoping it could be forced through the needle by pressing the plunger. The solution was difficult to expel: its viscosity was still too high for the electrospinning process. Under normal circumstances, I would have abandoned the experiment and simply gone home. But since I was already in the lab on Christmas Eve, I decided to proceed despite the viscosity problem. The prospect of returning home without completing the experiment was not appealing - especially given that my home would soon be without power again. |

Polymethyl methacrylate and polystyrene |

Dissolving Polymethyl Methacrylate (PMMA) in Methylene Chloride |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
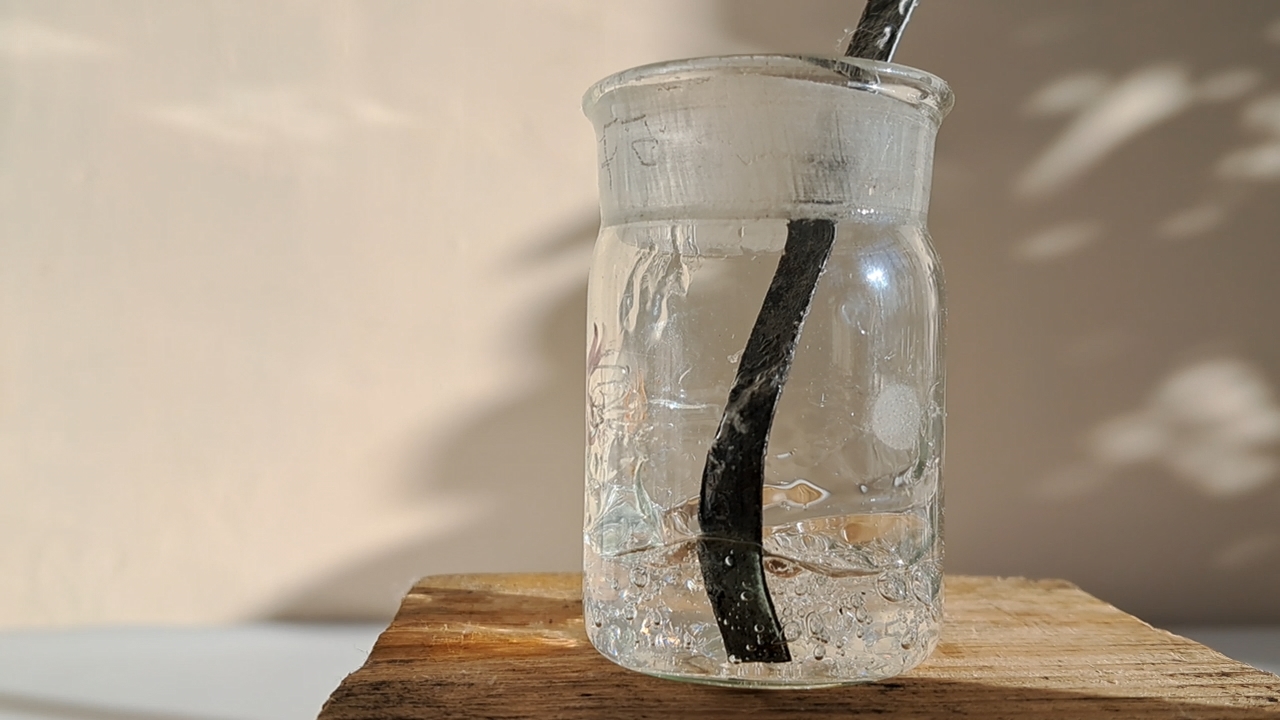
|

|

|

|
|
Having noticed a mistake in the text, allocate it and press Ctrl-Enter
Electrospinning: Solution of Polymethyl Methacrylate (PMMA) in Methylene Chloride - Part 19
Previous experiments had yielded two types of results: negative ones, in which an aerosol formed, and positive ones, in which fibers formed and covered the collector with a continuous layer. This experiment showed that reality is richer than such a binary classification.
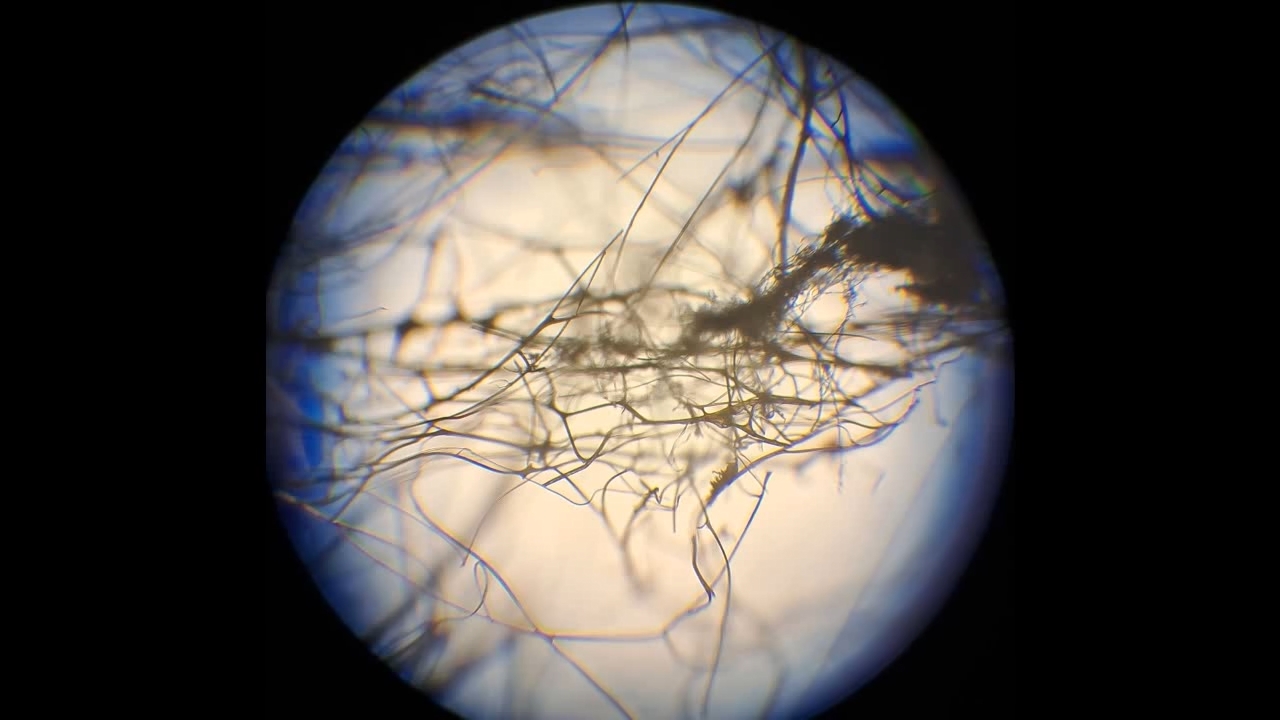
Электроспиннинг: раствор полиметилметакрилата (PMMA) в метиленхлориде - Часть 19 The experiment began much like the successful experiments with polystyrene. Shiny polymethyl methacrylate fibers shot out of the needle and rushed toward the collector. Soon, a "beard" of fibers formed, connecting the needle and the collector. It was already evening, and darkness had fallen. I turned off the lights in the room and filmed using my phone's flashlight, since daylight often interfered with the footage. The fibers shooting out of the needle glittered in the flashlight beam, making them clearly visible. A successful experiment? Not quite. Fibers did form, but a continuous layer of electrospun polymethyl methacrylate did not appear on the collector. Instead, "beards" of fibers developed, and small clumps accumulated on the collector - but nothing more. The experiment ultimately produced only a small clump of white, cotton-like material. Microscopic examination revealed that the fibers had a large diameter. Even without a microscope, however, it was obvious that the fibers were too thick. When a colleague squeezed the lump a few days later, remarking, "They should be elastic," the fibers turned out to be brittle. The polymer concentration in the solution was low, while the fiber diameter was large; under these conditions, it is unsurprising that the total amount of material obtained was small. Polymethyl methacrylate fibers were produced, but this result had no practical value. I returned home late, expecting the power to be out at that hour. However, electricity remained on throughout the evening and most of the night - there were deviations from the power schedule in both directions. The next day was Christmas. I went back to the lab and diluted the working solution, reducing the polymethyl methacrylate concentration by half. The fibers obtained were just as thick as before. They again failed to form a continuous layer on the collector, and their quantity was significantly smaller than in the previous experiment. While filming in the dark, I turned off my smartphone's flashlight and noticed a glow on the screen: "purple flames" emanating from the tip of the needle. At first, I thought the light was invisible to the naked eye. Looking more closely, I realized that the glow was visible, though faint and lacking detail - and only if one knew where and when to look. It was a corona discharge, accompanied by a hissing sound and the characteristic smell of ozone. Previously, I had noticed corona discharge only moments before a spark jumped between the electrodes. It turned out that corona discharge was occurring continuously during electrospinning; it was simply invisible in a well-lit room. It was likely that not only the needle tip but also the forming polymer fibers were glowing. Merry Christmas! |

Electrospinning: Solution of Polymethyl Methacrylate (PMMA) in Methylene Chloride |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
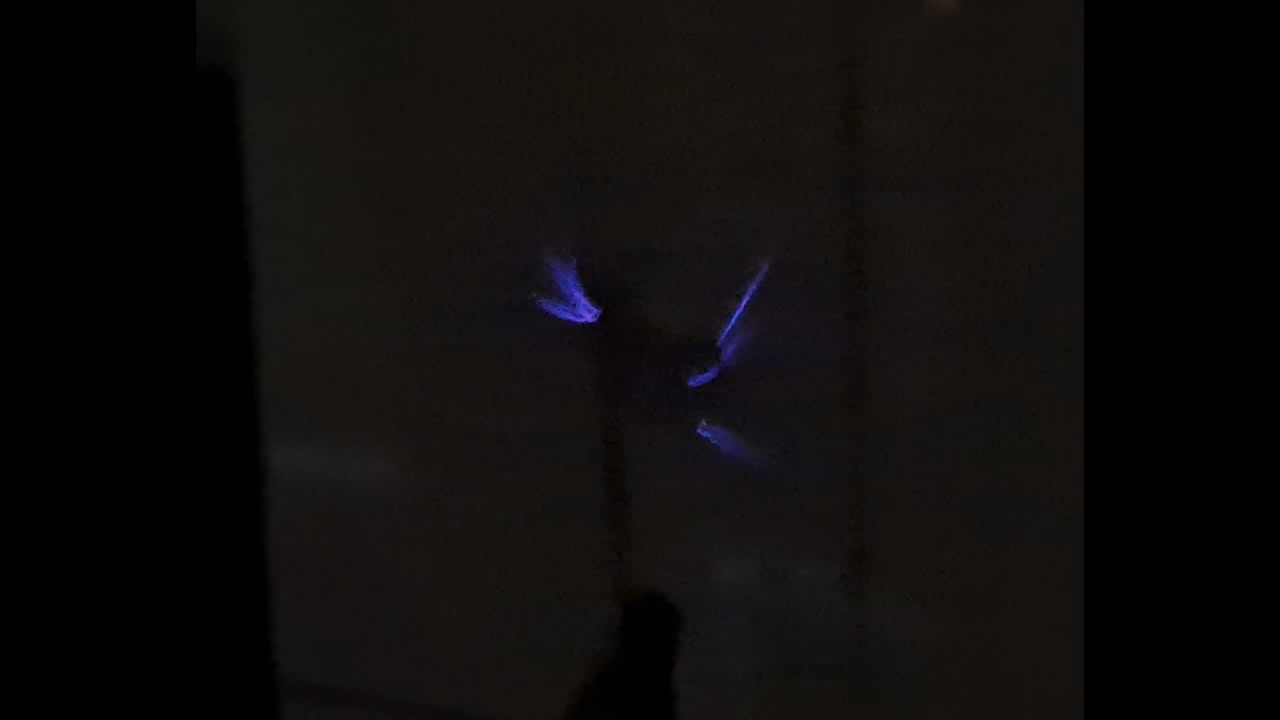
|

|

|

|

|

|

|

|

|

|

|
|

|
|
Комментарии
К1
Из доступных полимеров полистирол растворяется во многих растворителях.
Приклеивать пенопласт можно клеем серии БФ. Растворителем клея служит смесь этанола с диэтиловым эфиром.
Отсюда следует, что полистирол не растворим в этой смеси. Из других полимеров в ацетоне растворим перхлорвинил, но растворимость не велика, в основном набухает. Перхлорвинил - это "хлориновая ткань" и фильтры некоторых респираторов и масок. Этот вид фильтрующего материала разработан под руководством Петрянова-Соколова - "фильтр Петрянова", волокна довольно мелкие. При прохождении воздуха через фильтр волокна электризуются и пыль притягивается электростатикой - ИМХО. |