Chemistry and Chemists № 2 2024
Journal of Chemists-Enthusiasts
| Content | Chemistry experiments - video | Physics experiments - video | Home Page - Chemistry and Chemists |
|
Chemistry and Chemists № 2 2024 Journal of Chemists-Enthusiasts |
Experiments with perchloric acid and perchlorates - part 1, 2 Good Chemist |
|
Having noticed a mistake in the text, allocate it and press Ctrl-Enter
Determination of perchloric acid - qualitative reaction for perchlorate anion (potassium perchlorate precipitation) - part 1
"A long time ago in a galaxy far, far away" microbiologists needed... ammonium perchlorate NH4ClO4. They asked me to synthesize it. I wish to have told them: "Dear colleague, buy ammonium perchlorate. I do not want to bother with synthesising such a simple substance!" However, I remembered that ammonium perchlorate is an oxidizer used in rocket fuel, including in combat ballistic missiles. Therefore, the compound is unlikely to be sold freely. Even if it is for sale, such a purchase may trigger a visit from an investigator. Of course, a few tens of grams is not enough "to make a ballistic missile in the garage", but rationalism does not rule the world.
Эксперименты с хлорной кислотой и перхлоратами Идентификация хлорной кислоты - качественная реакция на анион перхлората (осаждение перхлората калия) - часть 1 I said: "I'll try, but I don't promise." How can it be synthesized? I looked in many practical books on inorganic chemistry but found nothing. By the way, the procedure for synthesizing ammonium chlorate (NH4ClO3), an extremely unstable and explosive substance, is described. However, any practical methods of ammonium perchlorate preparation are absent in these books. I found useful information about the synthesis of ammonium perchlorate only on amateur websites and YouTube channels. People have used sodium perchlorate and ammonium chloride (exchange reaction). I decided to do something simpler - obtaining perchlorate by reacting perchloric acid and ammonia solution. As a result of the search, I found in the laboratory as many as two old bottles with the labels "Perchloric acid". One of them was produced in 1977 (almost my age). The other bottle was less ancient (30 years "younger" than the first), but also not new. Do the contents match the labels? There was no one to ask. My laboratory neighbour is an 80-year-old man. At this time, he was sleeping peacefully right at his workplace. I did not wake him up - It is unlikely that the elderly man knew about the existence of these bottles. 
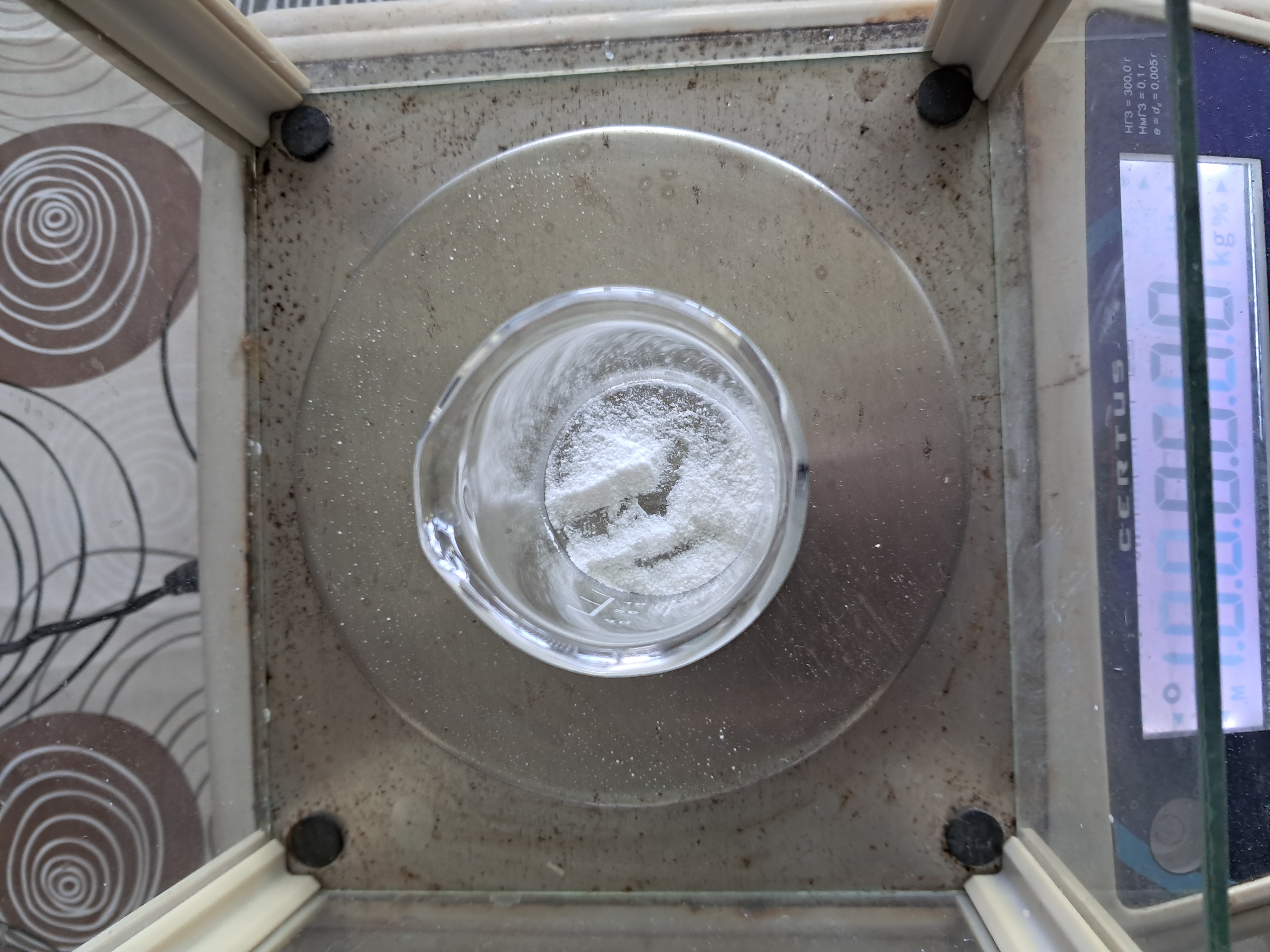
I poured the solution of potassium chloride into two glasses. Then, I added a small portion of the liquid from the first bottle to one glass, and a little liquid from the second bottle to the second glass. A white precipitate formed in both beakers. This means that the bottles contained perchloric acid. The precipitates were filtered, washed with distilled water, and dried. Perhaps I will conduct some kind of experiment with potassium perchlorate (the interesting compound should not go to waste). |
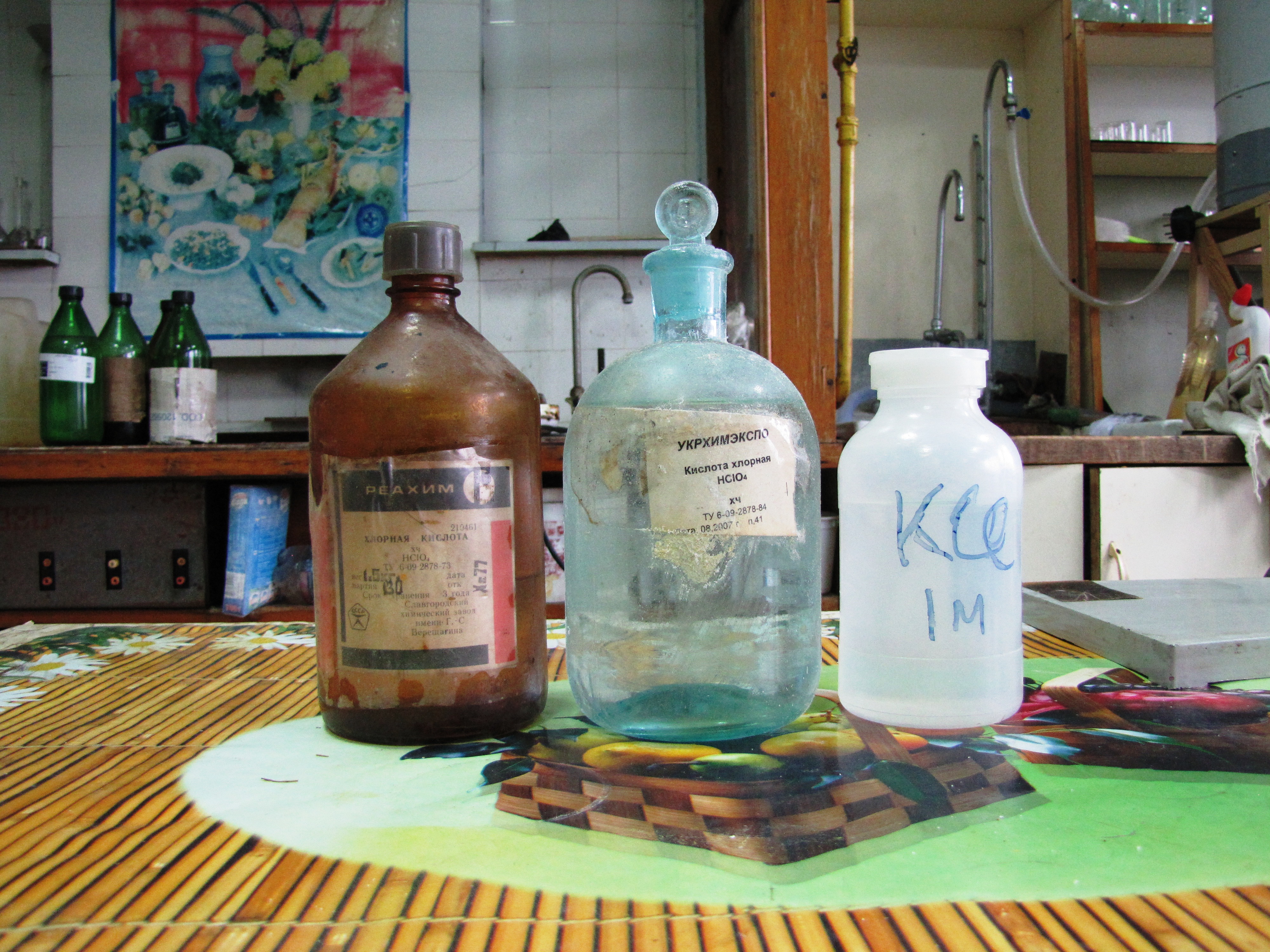
Perchloric acid |

|

|

Determination of perchloric acid - qualitative reaction for perchlorate anion (potassium perchlorate precipitation) |

|

|

|

|

|

|

|

|

|

|
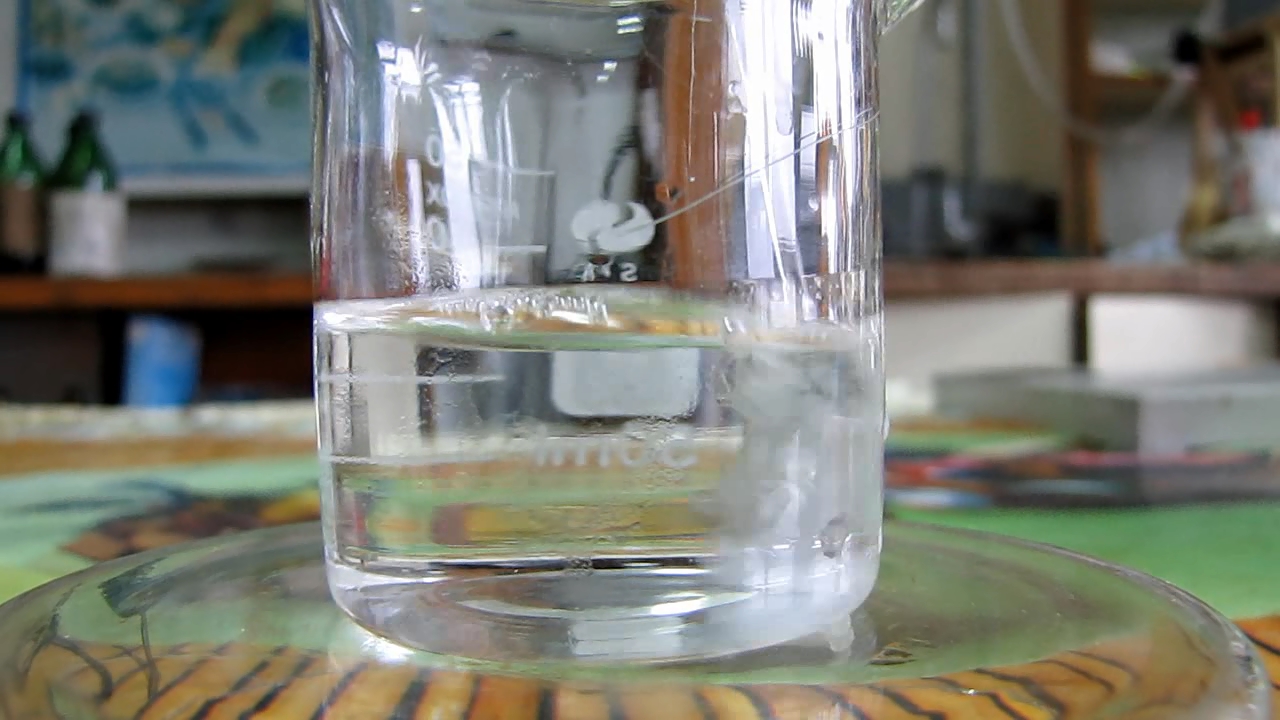
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
A little later, I found a flask in a secluded place with the inscription "HClO3" (chloric acid). There was a colourless liquid inside. I did not add or wipe anything away (not even dirt) and posted the photos as is.
Even if it is perchloric or hydrochloric acid, it is better to pour it out, because... the purity of the reagent is questionable. I am just wondering what the person who wrote this meant. Perhaps they added "O3" after "HCl" so that the laboratory would not have problems due to the so-called "precursors" (Illicit drug precursors, for example, hydrochloric acid). In principle, I do not accept the idea that there was chloric acid HClO3 in the flask. When I have time, I will experiment with the contents of the flask. |
|
Чуть позже нашел в укромном месте колбу с надписью "HClO3" (хлорноватая кислота). Внутри была бесцветная жидкость. Ничего не дорисовывал и не вытирал (даже грязь) - выкладываю фотографии, как есть.
Даже, если это хлорная или соляная кислота, лучше ее вылить, т.к. чистота реактива вызывает сомнения. Просто интересно, что имел ввиду человек, который это писалю. Возможно, после "HCl" он дописал "O3", чтобы у лаборатории не было проблем из-за т.н. "прекурсоров" (прекурсоры наркотических веществ, - соляная кислота, например). Мысли, что в колбе была хлорноватая кислота HClO3, я не допускаю в принципе. Когда будет время, поэкспериментирую с содержимым колбы. |

|

|

Aluminium and ammonium perchlorate |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

Aluminium and ammonium perchlorate |

|

|
|

|

|

|

|

|

|

|
|

|

|

|

|

|

|

|
|
Комментарии
К1
Белый осадок с солями калия в водном растворе дадут также борфтористоводородная кислота и её соли, кремнефтористоводородная кислота.Разница в осадках: фтороборат и фторсиликат калия будут растворяться в растворе щёлочи за счёт гидролиза аниона, а перхлорат - нет. Советский реактив "Хлорная кислота" имел концентрацию 57%. Критерий этой цифры - видимо, взрывчатость. Концентрацию легко определить по плотности. |