Chemistry and Chemists № 2 2024
Journal of Chemists-Enthusiasts
| Content | Chemistry experiments - video | Physics experiments - video | Home Page - Chemistry and Chemists |
|
Chemistry and Chemists № 2 2024 Journal of Chemists-Enthusiasts |
Experiments with perchloric acid and perchlorates - part 3 Good Chemist |
|
Having noticed a mistake in the text, allocate it and press Ctrl-Enter
I liked the reaction of the mixture of aluminium powder and ammonium perchlorate, so I decided to conduct further experiments with perchlorates. After some consideration, I chose to use titanium hydride (TiH2) and mix it with ammonium perchlorate, intending to ignite the mixture.
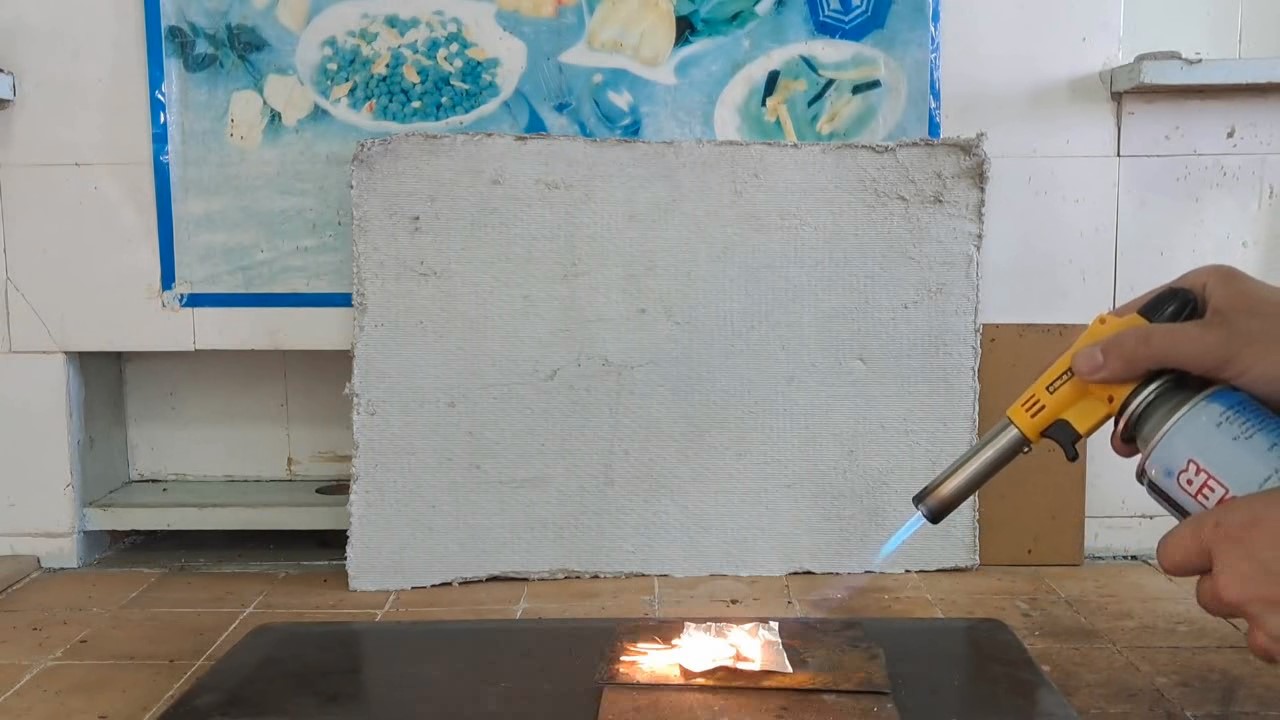
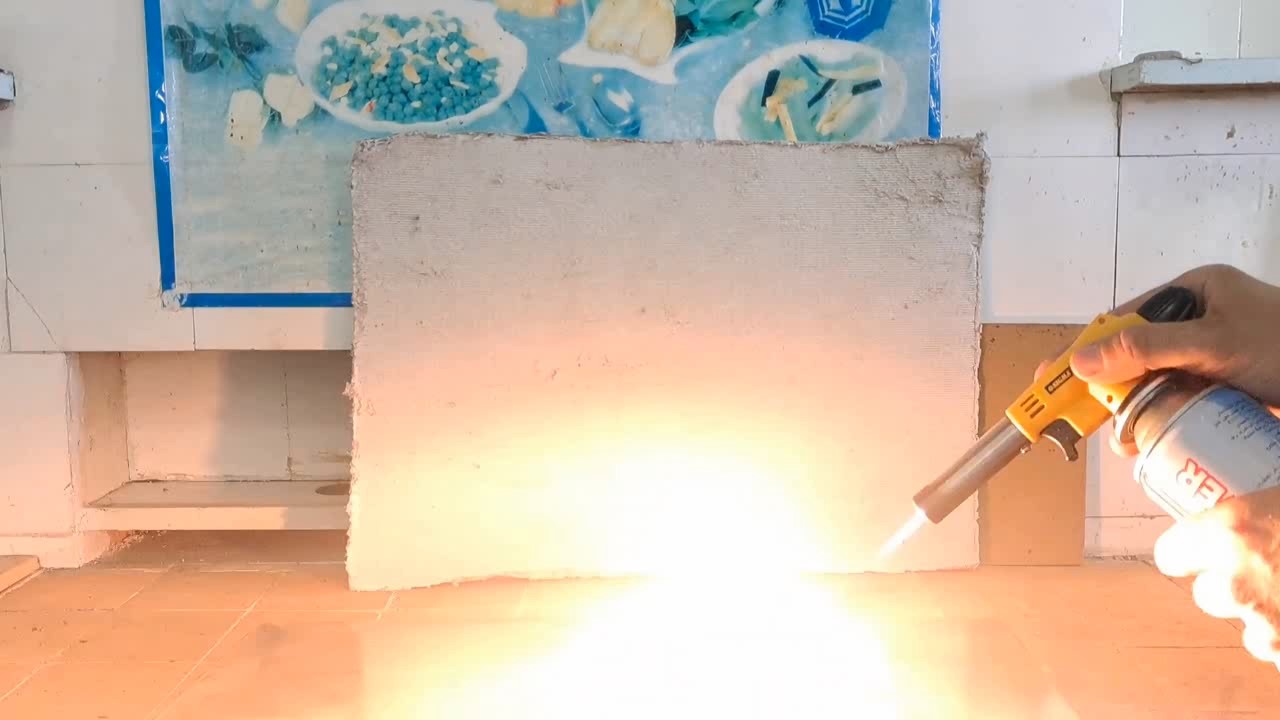
A colleague from another institute unexpectedly came by. Now he works as a physicist, but before that, he worked as a pyrotechnician for many years. Having heard that I would mix titanium hydride with an oxidizer, my colleague began to dissuade me from this experiment. - Titanium hydride reacts with water. Water may get into your mixture! This is dangerous! Perhaps even the moisture in the air will be enough to initiate this mixture. Indeed, some hydrides react actively with water and air. For example, sodium hydride, lithium hydride, and complex lithium aluminium hydride ignite or explode upon contact with water. Gaseous boron, silicon, and phosphorus hydrides spontaneously ignite in the air. However, titanium hydride does not react actively with water or air at room temperature. Unlike the substances mentioned earlier, titanium hydride is a metallic hydride (or alloy-like hydride), which behaves chemically like metals of medium reactivity. It is written that titanium hydride is resistant to water and air at room temperature. It reacts slowly with hydrochloric and sulfuric acids. To avoid offending my colleague, I chose not to argue but instead conducted an additional experiment to demonstrate that water does not initiate a reaction between ammonium perchlorate and titanium hydride. Furthermore, after adding water, the mixture can be dried and ignited. If the dried mixture still produces a flash, this will indicate that titanium hydride remains unchanged after treatment with water. First, I weighed 0.74 g of ammonium perchlorate and 0.26 g of titanium hydride. I mixed the substances, carefully ground them, and placed them on aluminium foil. I ignited the mixture with a gas burner. A bright yellow flash occurred (or rather, a series of instantaneous flashes). For the second experiment, I prepared a mixture of 0.50 g of ammonium perchlorate and 0.50 g of titanium hydride. This mixture also produced a bright yellow flash. In the third experiment, I prepared the same mixture (0.50 g ammonium perchlorate and 0.50 g titanium hydride), placed it in a glass, added 4 drops of water, stirred it, and left the mixture for a day. No signs of reaction were observed. After a day, the mixture was dried. I placed it on aluminium foil and set it on fire. A series of bright yellow flashes occurred, similar to those observed with the mixture that was not exposed to water. |

Titanium hydride and ammonium perchlorate |

|

|
|
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|