Chemistry and Chemists № 2 2025
Journal of Chemists-Enthusiasts
| Content | Chemistry experiments - video | Physics experiments - video | Home Page - Chemistry and Chemists |
|
Chemistry and Chemists № 2 2025 Journal of Chemists-Enthusiasts |
Experiments with Nitrocellulose - pt.1, 2 Chemist |
|
Having noticed a mistake in the text, allocate it and press Ctrl-Enter
Dissolving Nitrocellulose in Low-Quality Acetone - Part 1
My childhood memories prompted me to experiment with nitrocellulose. I used to drip glue onto the surface of water, creating a man-made "amoeba" that moved as if it were alive. I also have vivid memories associated with nitrocellulose from my time in the army, in a combat zone. Back then, I deliberately lit smokeless powder on my palm, imitating the famous experiment with nitrated cotton wool. My commander warned me against this, predicting that I would burn myself. I had also anticipated a similar outcome but chose not to stop. And indeed, I burned myself. The burn was painful and took a long time to heal. Unlike small amounts of black powder or nitrated cotton wool, smokeless powder causes painful burns when ignited on the skin.
Эксперименты с нитроцеллюлозой Растворение нитроцеллюлозы в некачественном ацетоне - часть 1 I will use the term ''nitrocellulose'' throughout the article, without always specifying that smokeless powder of the Sokol (''Falcon'') brand, rather than pure nitrocellulose, was used in the experiments. According to the literature, this powder consists mainly of nitrocellulose, though it includes other components as well. By "poor-quality" acetone, I mean acetone bought from a supermarket, which is heavily diluted with other solvents, likely hydrocarbons based on their behavior. To make nitrocellulose varnish, I needed nitrocellulose, which is produced by treating cotton wool with a mixture of concentrated sulfuric and nitric acids in a process called nitration. While it was possible to nitrate cotton wool in our laboratory, I chose not to, as concentrated sulfuric acid is difficult to purchase legally in our country. Instead, I decided to dissolve smokeless powder in acetone. At that time, I was unaware that the acetone I had was of poor quality. I weighed 1 g of smokeless powder, placed it in a glass weighing bottle, added 10 ml of acetone, and stirred with a glass rod. The nitrocellulose grains stuck together and swelled rather than dissolved, turning the liquid yellow. I sealed the bottle with a hermetic lid and left it for a while, occasionally opening the lid to stir the contents. Over time, the nitrocellulose swelled into a viscous, gel-like mass, with a mobile liquid layer remaining above it. Eventually, the nitrocellulose absorbed all the liquid, forming a green gel. When I tilted the weighing bottle horizontally, the contents showed no fluidity. I added another 10 ml of acetone and stirred again. The acetone turned yellow, and the liquid and gel separated. After about 24 hours, with periodic stirring, a homogeneous, viscous green liquid formed. Notably, pure nitrocellulose and its solution in acetone are colorless. The experiment was conducted during the summer, with laboratory temperatures of 20-25°C, so I did not consider heating the mixture. However, when I repeated the experiment in winter, with the laboratory at 4°C, I placed the weighing bottle in a thermostat set to 40°C. Under these conditions, the nitrocellulose dissolved much faster, taking about an hour. Unfortunately, the video documentation of the dissolution process was not of the best quality, as it was challenging to perform the experiment and record it simultaneously. |

Dissolving Nitrocellulose in Low-Quality Acetone |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
Having noticed a mistake in the text, allocate it and press Ctrl-Enter
Nitrocellulose Amoeba (Trial Experiment) - Part 2
The nitrocellulose solution was obtained, allowing me to repeat the "Artificial Amoeba" experiment that I performed as a child. At that time, I did not know that counterfeit acetone had been used to dissolve nitrocellulose, so I enthusiastically proceeded with the experiment.
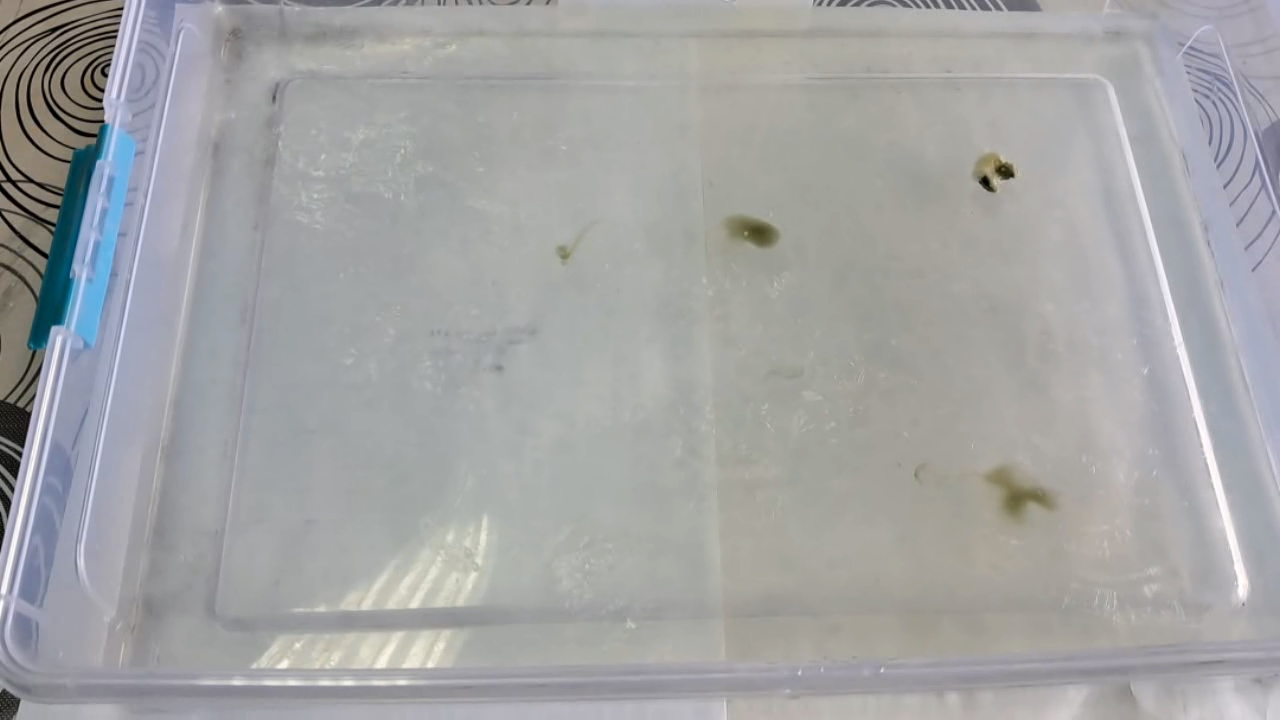
Амеба из нитроцеллюлозы (пробный эксперимент) - часть 2 First, I poured water into a Petri dish and dropped the nitrocellulose solution into it. I expected the drop to move chaotically along the surface due to the acetone "jets" being expelled from the drop into the water. This was exactly how the drop of nitrocellulose glue behaved when I used it as a child to imitate an amoeba. When the drop hit the surface of the water, it began to move like an amoeba. However, its movement did not last long: soon, the "amoeba" stopped. The reason was obvious - part of the nitrocellulose solution had spread over the surface of the water, forming a thin film. The "amoeba" became entangled in this film, like a fly in a web. Interestingly, in this case, the "web" was not woven by a spider - the "fly" had woven the "web" for itself. I removed the film with a glass rod. This helped briefly: the drop began to move again, but soon another film formed, and the drop got stuck once more. I repeated the process several times, trying other Petri dishes and even using a large tray of water instead. The result was similar: in a plastic tray, the "amoeba" moved for longer than in a Petri dish because of the larger surface area, but eventually, a film formed, and the "amoeba" stopped. Removing the nitrocellulose film from the surface helped only temporarily or not at all. Only once was I able to achieve an "amoeba" that moved quickly and for a long time. What caused the failure of the experiment? At first, I assumed that the smokeless powder contained additives that interfered with the experiment, causing the result to differ from what I had observed years ago. Later, I discovered that the solvent used was not pure acetone - it contained a large fraction of hydrocarbons. Since hydrocarbons are insoluble in water, they spread over its surface, forming a film. After the hydrocarbons evaporated, a thin film of nitrocellulose remained. A few months later, I learned that an "artificial amoeba" could be produced even using our low-quality acetone, but I will save that story for another time. |

Nitrocellulose Amoeba (Trial Experiment) |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|

|

|

|