Chemistry and Chemists № 2 2025
Journal of Chemists-Enthusiasts
| Content | Chemistry experiments - video | Physics experiments - video | Home Page - Chemistry and Chemists |
|
Chemistry and Chemists № 2 2025 Journal of Chemists-Enthusiasts |
Experiments with Nitrocellulose - pt.3, 4 Chemist |
|
Having noticed a mistake in the text, allocate it and press Ctrl-Enter
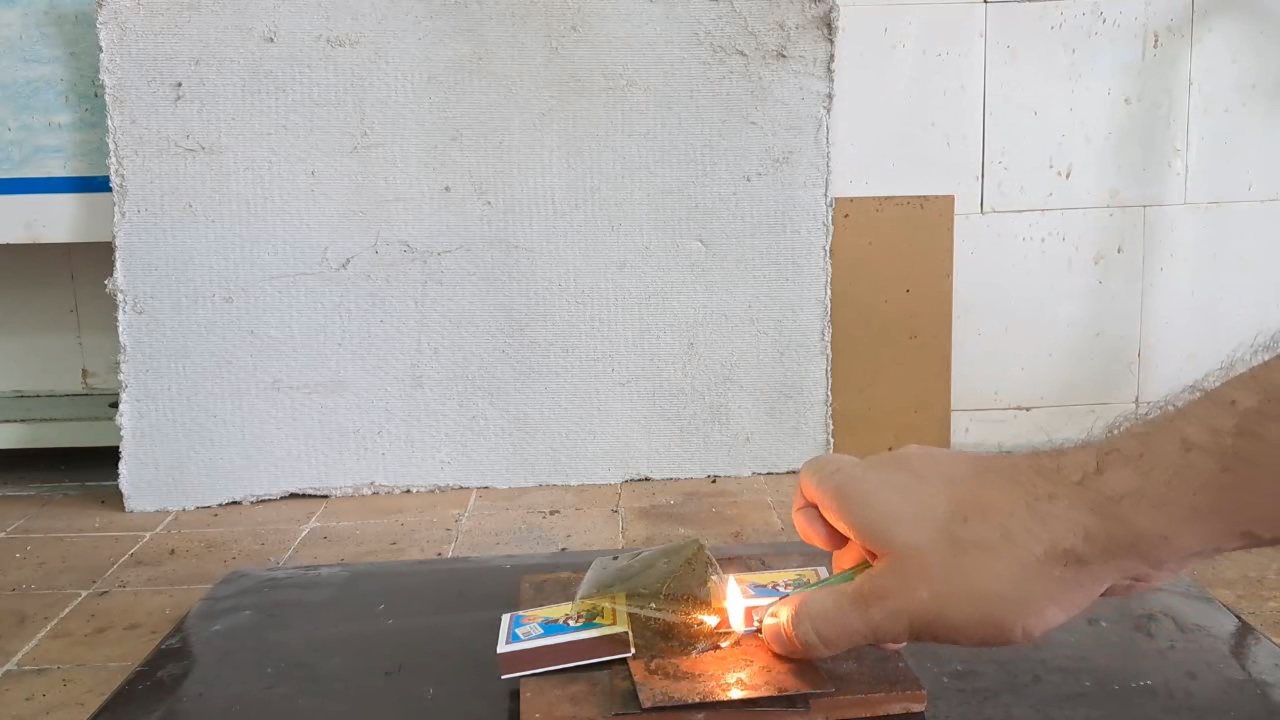
In the previous experiment, a nitrocellulose solution formed a thin film on the surface of water. This gave me the idea of obtaining a nitrocellulose film to demonstrate its combustion. To achieve this, I poured the previously prepared nitrocellulose solution in acetone into a dry Petri dish, spread it over the surface with a glass rod, and allowed the solvent to evaporate. As I feared, the nitrocellulose film adhered to the glass and could not be separated with the rod. I then poured water into the Petri dish and left it for a while. Eventually, the film detached from the glass and floated to the surface of the water. I carefully removed the nitrocellulose film from the dish and dried it.
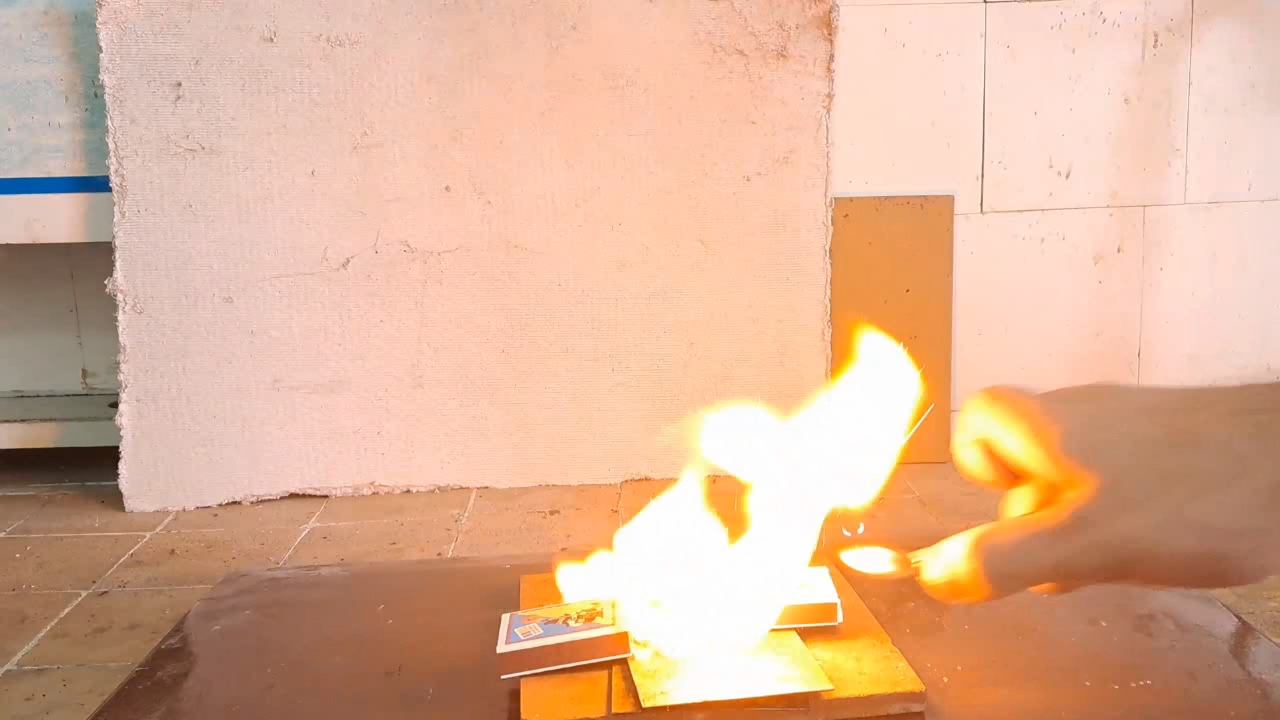
When ignited, the film burned with a yellow flame in about a second. This reminded me of an experiment I once conducted during my time in the army, where I attempted to burn nitrocellulose on the palm of my hand. At the time, the commander tried to dissuade me, but I didn't listen - and ended up getting burned. While reflecting on that experience, I recalled that the village where we were stationed is now under occupation. At that moment, the head of the laboratory entered the room. After learning what I was planning to do, he also tried to dissuade me: "- You'll burn your hand!" After some thought, I decided to proceed, reasoning that I wasn't dealing with smokeless powder granules this time, but a thin nitrocellulose film. I acknowledged the risk of getting burned, but I doubted it would be severe. I placed the film on my palm and lit it with a lighter. There was a quick yellow flash, and I felt a strong burning sensation. As soon as the combustion ended, I instinctively pulled my hand away. It was painful, but fortunately, I didn't suffer any burns. |

Combustion of Nitrocellulose Film |

|

|

|

|

|

|

|

|
|

|

|

|
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

Combustion of Nitrocellulose |
|

|

|

|

|

|
|

|

|
|

|

|

|
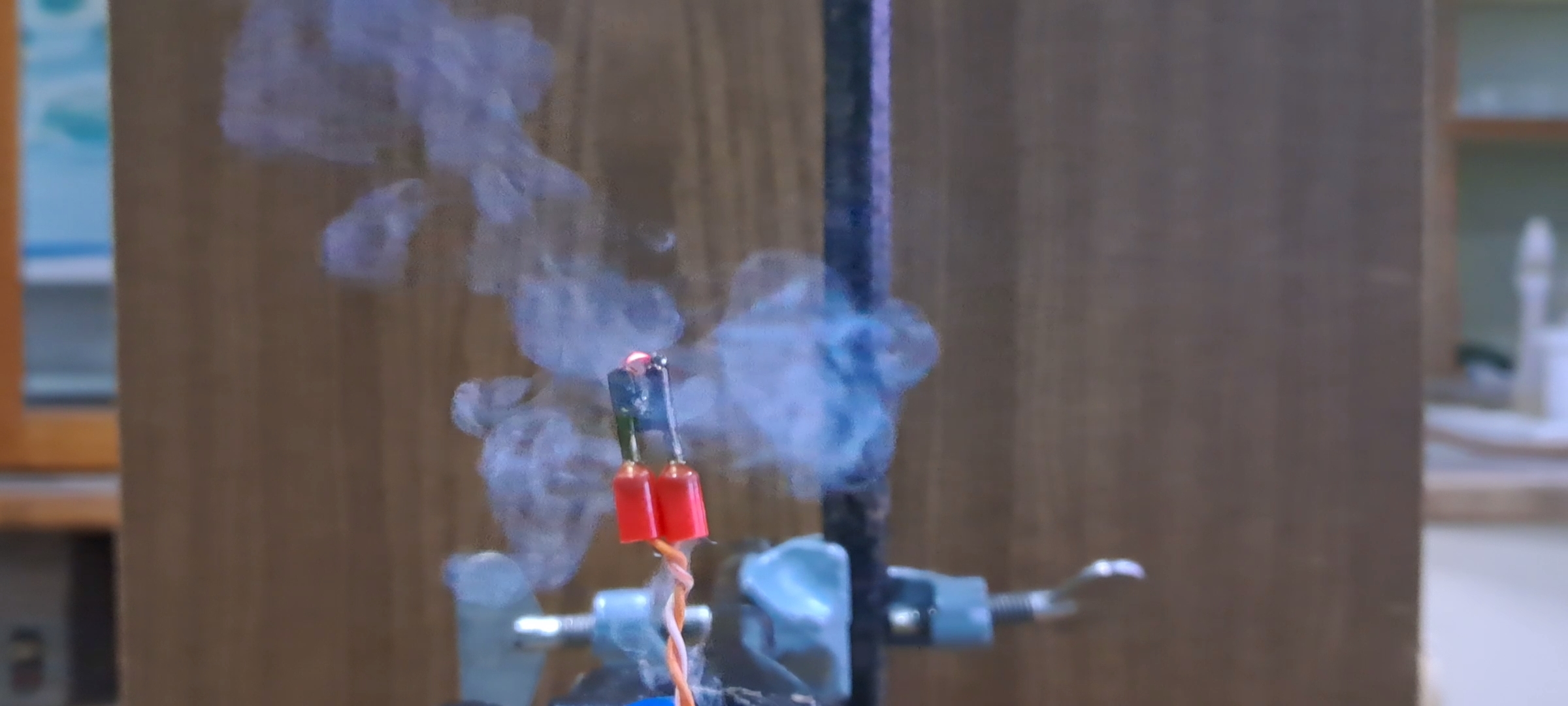
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

(The polystyrene solution) |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
Комментарии
К1
В конце 70-х я работал примерно год в НИИ прикладной математики и механики при Томском гос.университете лаборантом. Готовил эксперименты по изучению скорости горения разных составов в зависимости от давления азота от вакуума до примерно 100 атм.При низком давлении (под колоколом) нитроцеллюлоза, октоген и разные смеси не горят. Видимо, это представляет проблему в открытом космосе. При повышенном давлении азота скорость горения увеличивается. |