Chemistry and Chemists № 2 2025
Journal of Chemists-Enthusiasts
| Content | Chemistry experiments - video | Physics experiments - video | Home Page - Chemistry and Chemists |
|
Chemistry and Chemists № 2 2025 Journal of Chemists-Enthusiasts |
Experiments with Nitrocellulose - pt.8, 9 Chemist |
|
Having noticed a mistake in the text, allocate it and press Ctrl-Enter
Nitrocellulose Amoeba (Low-Quality Acetone) - Part 8
An interesting idea arose during the previous experiment. It turned out that an artificial "amoeba" might not form even when using high-quality acetone if the viscosity of the nitrocellulose solution is insufficient. This fact allows us to suggest that the experiment could succeed even with low-quality (fake) acetone, provided the nitrocellulose solution is viscous enough.
Нитроцеллюлозная амеба (некачественный ацетон) - часть 8 To test this, I prepared a viscous nitrocellulose solution in low-quality acetone and dropped it into a crystallizer filled with water. Initially, the "amoeba" moved, but a thin film quickly formed around it, preventing further movement. The drop stopped. Removing the film initially had no effect, as a new film formed immediately. However, after consistently removing the film as it appeared, new films eventually stopped forming after 20-30 seconds. The "liberated amoeba" then began moving across the entire surface of the water. The movement continued for about 10 minutes, albeit more slowly than in the previous experiment with high-quality acetone. Nonetheless, the amoeba's movement lasted for quite a long time. Unfortunately, the reproducibility of this experiment is limited. Pure substances, such as acetone, have well-defined physical properties that only vary slightly depending on impurities. In contrast, surrogates often have different chemical compositions depending on how they were prepared. For instance, manufacturers may dilute acetone with various solvents in differing quantities, resulting in unpredictable behavior. As a result, using other batches of low-quality acetone could yield entirely different outcomes. Conclusion. Experiments should ideally be conducted with pure chemicals or with mixtures of substances of known and reproducible composition. Exceptions may be made temporarily if pure substances are unavailable. |

Nitrocellulose Amoeba (Low-Quality Acetone) |

|

|

|

|

|

|

|

|
|
Having noticed a mistake in the text, allocate it and press Ctrl-Enter
Nitrocellulose Amoeba and Aluminum Powder - Part 9
In addition to adding dye to a nitrocellulose solution, there are other ways to visualize the acetone jets that "shoot" from a drop of solution placed on the surface of water. The idea struck me when I recalled a science fiction film based on the novel Solaris by the Polish writer Stanisław Lem. The story unfolds on a distant planet, which is depicted as a vast, intelligent ocean. Interestingly, three decades passed between the novel's publication in 1961 and the discovery of the first exoplanets. At the time, it was not yet known that some planets could be water worlds.
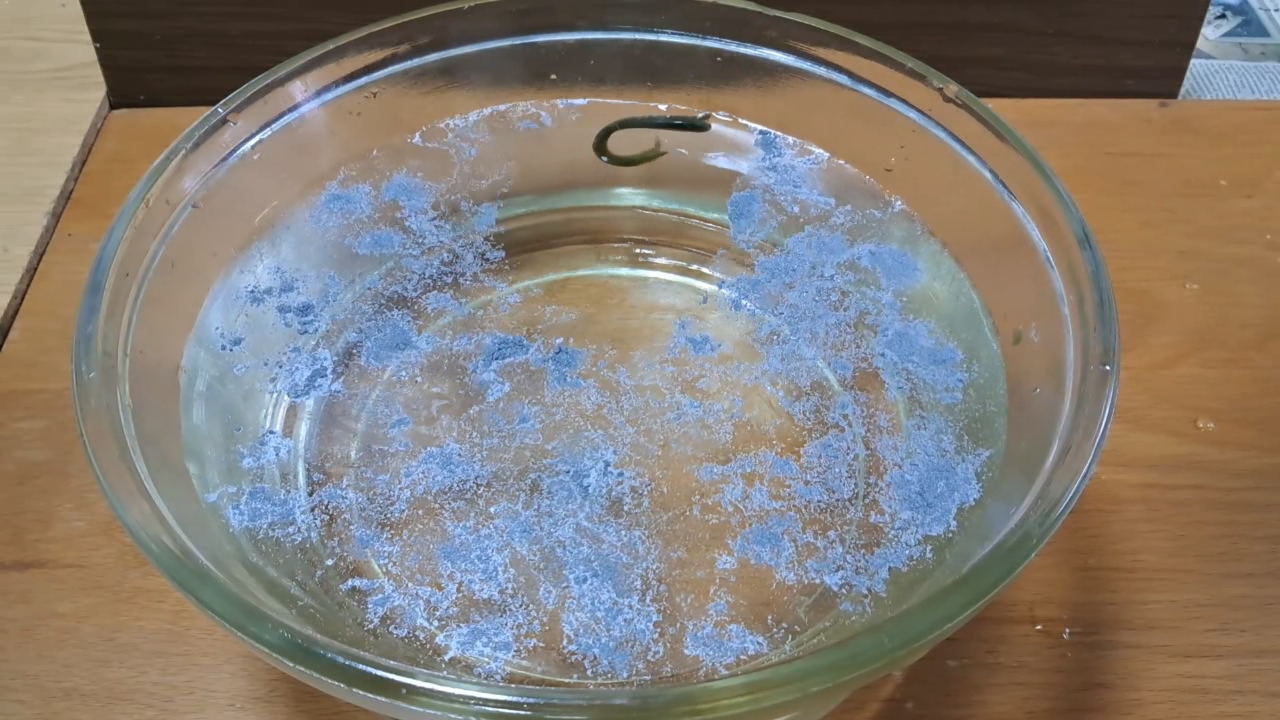
Нитроцеллюлозная амеба и алюминиевая пудра - часть 9 The film, released in 1972, was created before computer-generated special effects became commonplace in cinema. To simulate the alien ocean, the filmmakers used an aquarium filled with acetone, to which dyes and aluminum powder were added.  Solaris (Tarkovsky, 1972) Lem himself was highly critical of this adaptation. Personally, I did not enjoy the film either, as it distorts the novel's core ideas and the spirit of the original work. However, the idea of using aluminum powder to visualize liquid flows proved surprisingly useful. I filled a crystallizer with water and sprinkled the surface with fine aluminum powder, commonly used as a pigment. Then, I dropped a viscous solution of nitrocellulose in low-quality acetone onto the water's surface. (I used low-quality acetone to avoid wasting the limited supply of high-quality acetone on preliminary experiments.) The first drop formed into a long, worm-like shape and began to move. The second drop, which took on an irregular shape, started to rotate. Around each drop, its movement and the flows of the solvent cleared solid particles from the water's surface. I initially expected that a thin film of nitrocellulose would form, spreading across the water and eventually halting the drops' motion. However, something unexpected occurred. The film did start to form, but the aluminum particles obstructed its spread across the water's surface. Instead of a wide, thin film, a thick film developed, encapsulating the drop. This thicker film not only failed to slow the drop but became part of it. Acetone jets emerged from this film, enabling the "amoeba" to continue its active motion. Over time, the drops assumed an irregular, circular shape spanning several square centimeters. These drops, surrounded by their thick films, resembled diagrams of plant cells found in biology textbooks more than they did amoebas. For comparison, I added two drops of nitrocellulose solution prepared with high-quality acetone to the crystallizer. This attempt produced a surprising yet less favorable result. The drops assumed worm-like shapes and began to move along the surface, pushing away aluminum particles and creating zones free of solid particles on the water's surface. However, the movement of these "amoebas" was slow and significantly hindered by the aluminum particles. Under these conditions, a solution of nitrocellulose in low-quality acetone proved to be far more effective for creating an "amoeba." Its partial spreading on the water surface increased the area from which acetone streams flowed into the water, enhancing the thrust of the jets. In contrast, the high-quality acetone formed "amoebas" that moved faster on a clean water surface but were substantially slowed by the aluminum particles. The limited surface area of these "amoebas" restricted their ability to generate sufficient thrust to overcome the surrounding aluminum particles. Repeated experiments yielded similar results. The "amoebas" moved actively for several minutes (low-quality acetone), while aluminum particles clearly visualized the streams of liquid jets shooting from their surfaces. This simple yet effective demonstration could be a valuable addition to lectures and public science demonstrations. |

Nitrocellulose Amoeba and Aluminum Powder |

|

|

|
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|