Chemistry and Chemists № 2 2025
Journal of Chemists-Enthusiasts
| Content | Chemistry experiments - video | Physics experiments - video | Home Page - Chemistry and Chemists |
|
Chemistry and Chemists № 2 2025 Journal of Chemists-Enthusiasts |
Experiments with Thermite - pt.22, 23 Chemist |
|
Having noticed a mistake in the text, allocate it and press Ctrl-Enter
Combustion of Thermite: Calcium Sulfate/Aluminum (CaSO4/Al) - Part 22
The most common oxidizer in thermite mixtures is iron oxide, a cheap and readily available substance. You can also use oxides of manganese, lead, nickel, copper, chromium, and some other metals. Even a thermite containing titanium oxide (TiO2) has been described. In addition to metal oxides, other oxidizers are sometimes included in thermites, such as sodium, potassium, strontium, and barium nitrates. Chromates, dichromates, chlorates, and perchlorates can also serve this purpose.
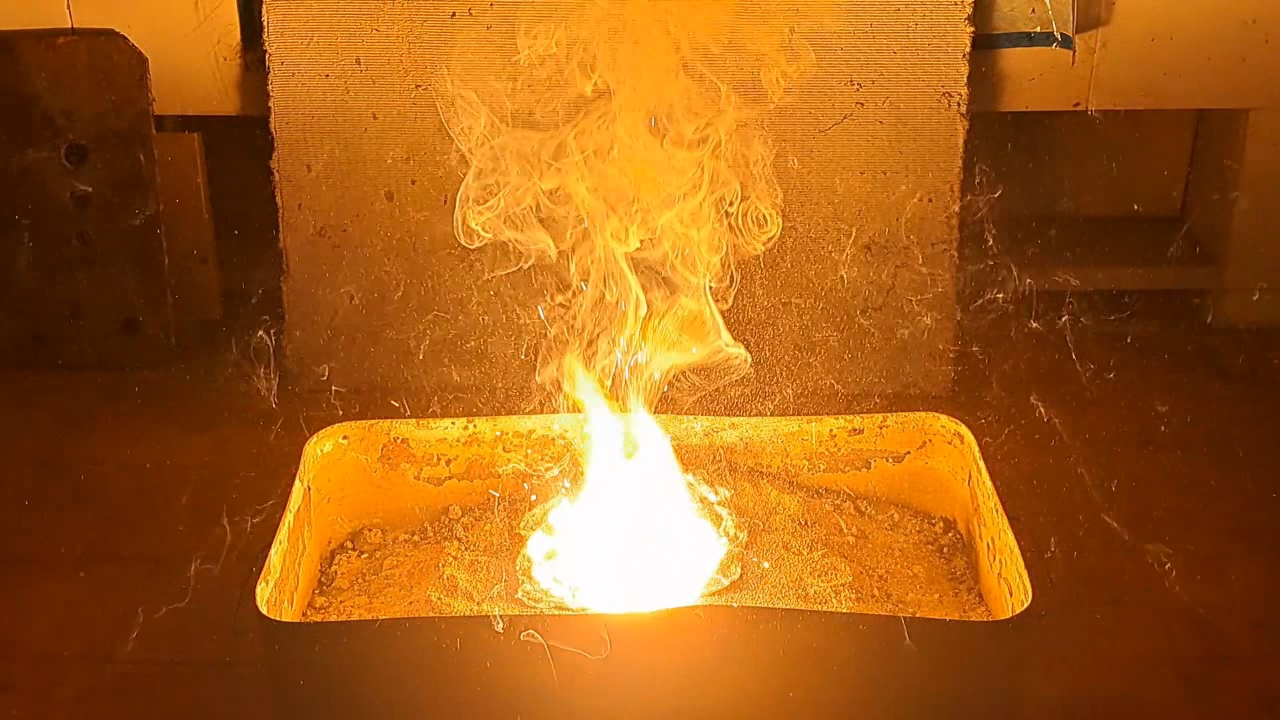
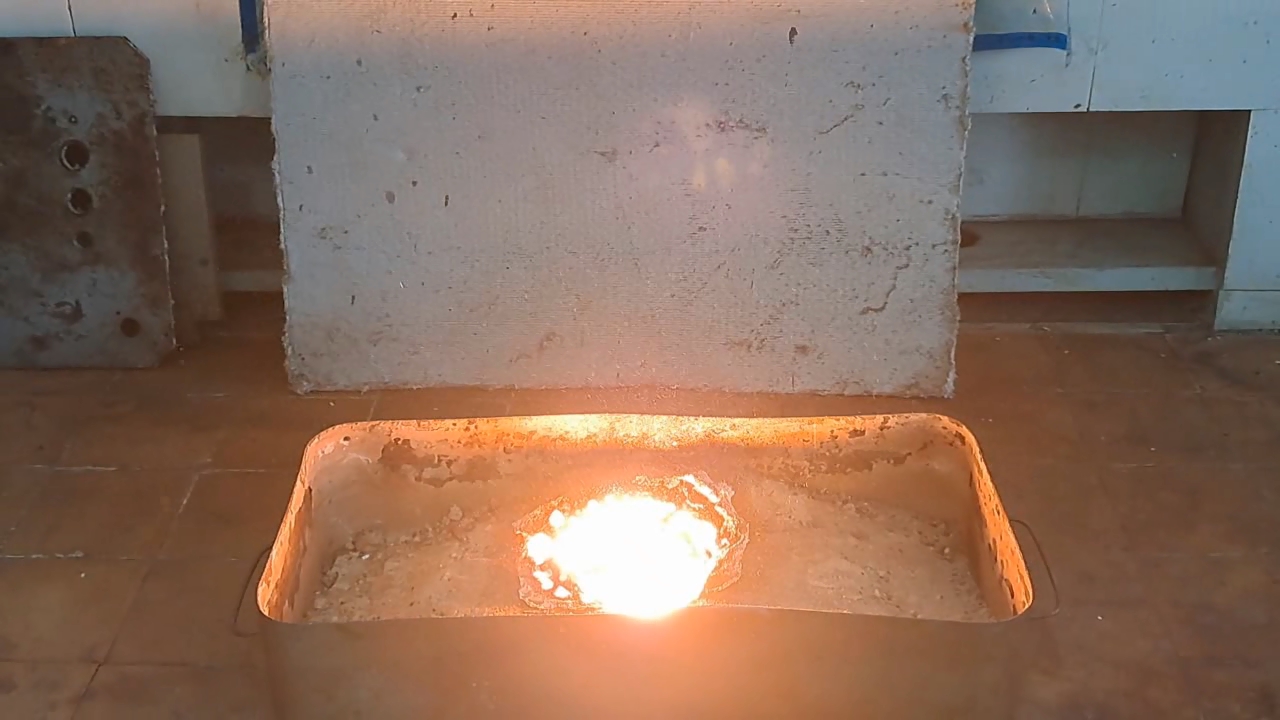
Горение термита: сульфат кальция/алюминий (CaSO4/Al) - часть 22 However, reality is sometimes stranger than our wildest fantasies. While reviewing thermite compositions, I came across the following (% by weight): 1. Al - 40.9, CaSO4 - 57.8, S - 1.0, castor oil - 0.3 2. FexOy - 59.2, Al - 25.3, BaSO4 - 15.3 In the first case, calcium sulfate acts as the oxidizer; in the second, barium sulfate serves as the oxidizer in combination with iron oxides. Calcium sulfate is a poorly soluble calcium salt used in construction, medicine, agriculture, and many other fields. When thinking of calcium sulfate, I usually imagine gypsum sculptures, gypsum stalactites in caves, building materials based on it, and even food additives. Sulfuric acid can also be produced from calcium sulfate, though this is not the most efficient method. However, I had never thought of sulfate as an oxidizer. I could recall only one example of such a reaction: calcium sulfate turning into sulfide when heated with carbon. Barium sulfate reacts similarly. Calcium sulfate precipitates from aqueous solutions as a dihydrate (CaSO4·2H2O), which occurs naturally as the mineral gypsum. When heated, gypsum loses water, becoming first the hemihydrate (CaSO4·0.5H2O), then anhydrous calcium sulfate. The hemihydrate is widely used in construction, architecture, and medicine. When water is added and stirred, it reverts to gypsum (CaSO4·2H2O), hardening into a solid mass. In the laboratory, I had some hemihydrate calcium sulfate, sold in our country as "alabaster" (also known as gypsum plaster or plaster of Paris). I decided to dehydrate it to prepare thermite. According to the literature, when calcium sulfate hydrates are heated above 250°C, anhydrous salt forms, which does not reabsorb moisture upon cooling. This is important for thermite, since water in the mixture can lead to splashing during the reaction. I poured the hemihydrate into a porcelain dish and placed it in a drying cabinet. I didn't dare to heat the cabinet to 250°C, fearing damage to the equipment, so I set the temperature to 230°C and heated for 2 hours. Next, I used the first thermite recipe, excluding sulfur and castor oil. I weighed 19.84 g of aluminum with 28.04 g of calcium sulfate (all the anhydrous salt obtained). I thoroughly mixed the powders in an iron mortar and poured them into a mound on a sheet of paper. The paper was placed in an iron tray filled with dry sand. I made a small depression at the top of the mound for the incendiary mixture. The thermite was a light gray powder, which was quite unusual. Not only the appearance, but also the composition was unconventional. I wasn't sure this thermite would ignite, so I decided to use double the usual amount of incendiary mixture. I combined 2 g of finely dispersed aluminum with 5.5 g of iron oxide (Fe3O4) and poured the mix into the depression. I aimed a burner flame at the incendiary mixture. Sparks rained down. After a few seconds, the mixture flared up, igniting the thermite. A huge, blindingly white flash occurred. Sparks flew in all directions with a furious hiss. In video, the flame appears yellow, but to the naked eye, it was dazzling white. The flash lasted about ten seconds, leaving behind a depression filled with a molten mass that slowly cooled. A large amount of acrid smoke was released, with a strong sulfur dioxide smell. The combustion resembled images and videos of the Sun's surface taken through powerful telescopes. Of course, the thermite's temperature was far lower than the Sun's surface temperature (about 5500°C), but the Sun is 151.4 million kilometers away—while the thermite was burning just a meter from me. The reaction produced a dark gray mass, part of which dispersed throughout the fume hood as fine particles. One of the products was calcium sulfide; aluminum sulfide was probably formed as well. Both compounds release hydrogen sulfide when exposed to atmospheric moisture, so I carefully collected the solid combustion products and sealed them in a jar. |
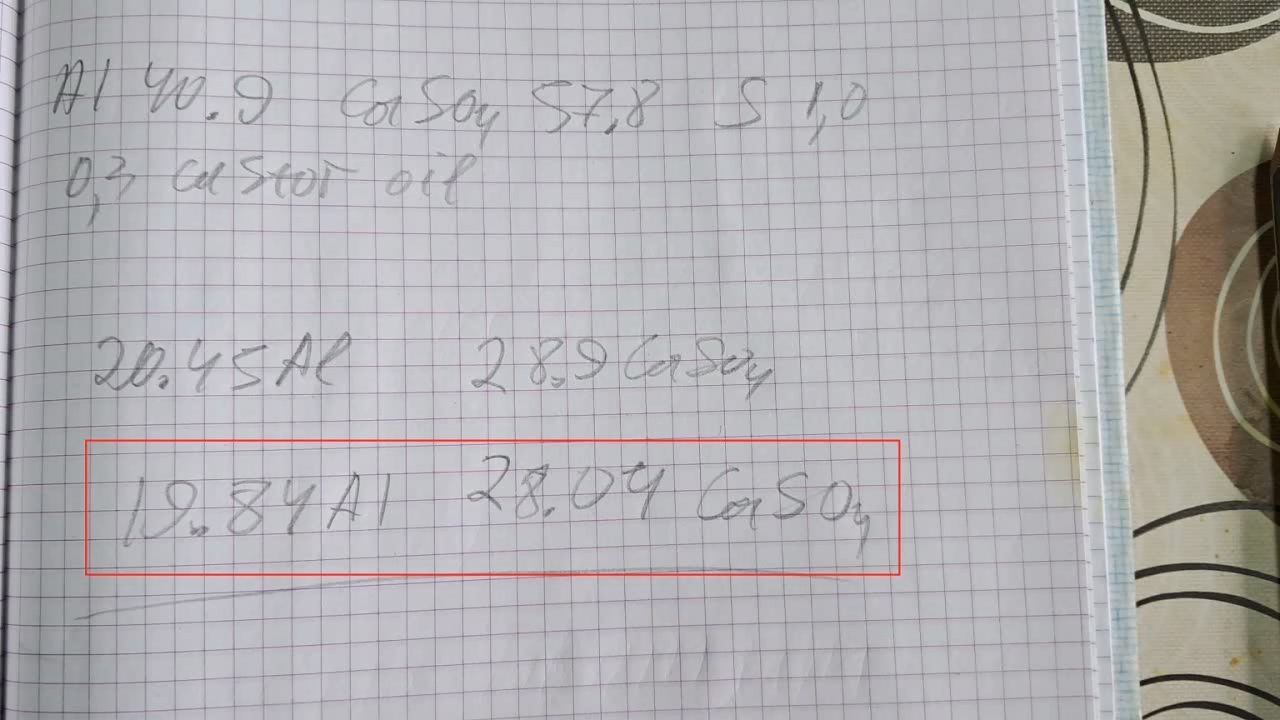
Combustion of Thermite: Calcium Sulfate/Aluminum (CaSO4/Al) |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|

|

|
|

|

|

|

|

|

|

|

|
|
Having noticed a mistake in the text, allocate it and press Ctrl-Enter
Combustion of Thermite: Calcium Sulfate/Aluminum (CaSO4/Al) - Part 23
In this experiment, I increased the amounts of aluminum and anhydrous calcium sulfate, mixing 42.5 g of Al with 60.0 g of CaSO4. The aluminum particle size was 100-140 microns. I poured the mixture into a cut aluminum beer can, which I placed in an iron tray filled with dry sand.
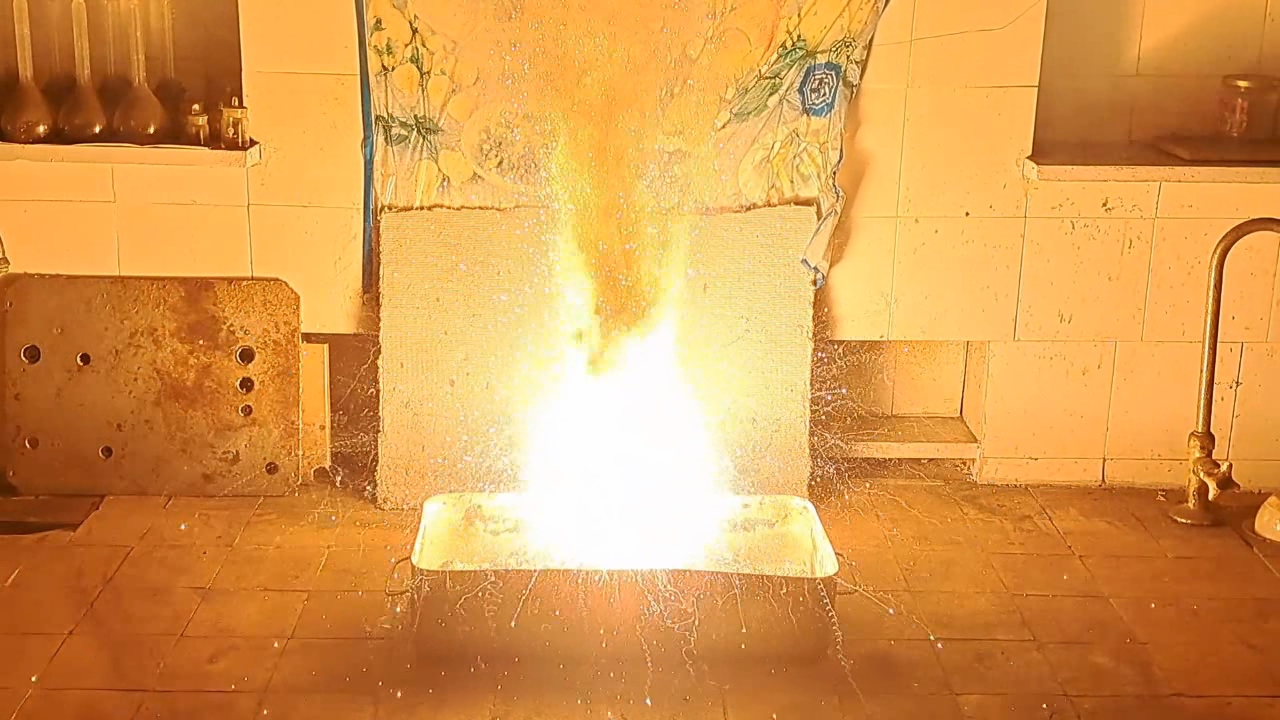
Горение термита: сульфат кальция/алюминий (CaSO4/Al) - часть 23 Based on the previous experiment, I was almost certain that this thermite mixture would ignite from a burner flame without the need for an incendiary composition. Therefore, I directed a strong gas burner flame at the top of the thermite mound. The mixture heated to a red glow, then to a yellow glow, but did not ignite. Conclusion: an incendiary mixture is necessary. After the mixture cooled, I made a depression in the thermite and placed an incendiary composition in it, prepared from 2 g of finely dispersed aluminum and 5.5 g of iron oxide (Fe3O4). I directed the burner flame at the composition. Sparks flew, small flashes were observed, and finally the incendiary mixture ignited. This was followed by a powerful, blinding white flash. Sparks shot in all directions with a hiss. Vigorous combustion continued for about 15 seconds, leaving behind a "lake" of molten material. A little later, a series of small but bright flashes occurred—the remaining mixture was burning out. As in the previous experiment, the combustion appeared yellow in video, but when observed directly, it was blindingly white. The combustion produced a large amount of acrid smoke with a strong sulfur dioxide odor. The polyethylene film with a printed image, which was glued to the wall of the fume hood, was severely damaged. In earlier experiments, many holes had already been burned through it by sparks. This time, a large hole burned through the film, making the image look very unaesthetic, so it had to be removed. Once the flashes ceased, a lake of molten residue remained, which gradually solidified into a gray solid mass. A significant portion of the solidified melt was scattered as small particles over the surface of the sand and around the fume hood. I collected as much of the solid reaction product as possible in a sealed jar, since it releases hydrogen sulfide upon exposure to air. Despite cleaning the fume hood, the lab continued to smell of hydrogen sulfide. The best solution would have been to replace the sulfide-contaminated sand in the tray with fresh dry sand, but none was available at the time. Therefore, I dissolved iron(III) chloride in water and poured the solution over the sand. Iron chloride reacts with hydrogen sulfide and soluble sulfides, converting them into insoluble iron sulfide. This measure helped only partially—the lab retained the odor of hydrogen sulfide for several days. |

Combustion of Thermite: Calcium Sulfate/Aluminum (CaSO4/Al) |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

Iron(III) chloride |

|

|