Chemistry and Chemists № 2 2025
Journal of Chemists-Enthusiasts
| Content | Chemistry experiments - video | Physics experiments - video | Home Page - Chemistry and Chemists |
|
Chemistry and Chemists № 2 2025 Journal of Chemists-Enthusiasts |
Experiments with Thermite - pt.28, 29 Chemist |
|
Having noticed a mistake in the text, allocate it and press Ctrl-Enter
Combustion of Thermite: Barium Sulfate/Aluminum (BaSO4/Al) - Part 28
For this experiment, I used technical-grade barium sulfate (commonly used in lead-acid batteries) and aluminum powder (particle size: 100-140 microns). Based on the reaction equation below, I performed the necessary calculations:
Горение термита: сульфат бария/алюминий (BaSO4/Al) - часть 28 8Al + 3BaSO4 = 4Al2O3 + 3BaS M(Al) = 26.98 g/mol M(BaSO4) = 233.38 g/mol m(Al) = 50 g m(BaSO4) = (50 x 3 x 233.38) / (26.98 x 8) = 162.19 g I thoroughly mixed 50 g of aluminum with 162.19 g of barium sulfate, placed the mixture into a cut aluminum beer can, and lightly compressed it. I made a depression in the center and added an incendiary composition consisting of 1 g of finely dispersed aluminum and 2.75 g of iron oxide (Fe3O4). As before, the experiment was conducted outdoors. I placed the can on a sheet of iron (specifically, the body of a gas mask filter housing, which turned out to have fairly thick walls). I directed a strong flame from a gas burner onto the incendiary mixture. It heated up to a red glow, and the aluminum wall of the can behind the flame melted and became wrinkled—but the main mixture did not ignite. This was likely due to insufficient mixing of the components. I loosened and stirred the incendiary mixture with a stick, then applied the flame again. This time, the incendiary composition ignited, and shortly afterward, the aluminum/barium sulfate mixture also ignited. A large, blinding white flame erupted. In the video, the flame appears yellow due to the camera's inability to capture colors accurately. Sparks flew in all directions. The combustion was accompanied by a loud hissing sound and produced a large amount of white smoke. The reaction left a red-hot circular zone on the iron sheet. Numerous small, solidified droplets of melt were scattered around the area. The iron sheet was burned through. I left the burned-through sheet outdoors in a secluded location. After a while, it began to emit the smell of hydrogen sulfide due to the reaction between barium sulfide and moisture in the air. The reaction product of barium sulfate and aluminum is barium sulfide, which is toxic. Therefore, inhaling the resulting smoke is extremely dangerous—another important reason why such experiments must never be performed indoors. In conclusion, I would like to highlight an important point. Pure barium sulfate is non-toxic and is used in medicine as a contrast agent for radiographic imaging. Patients ingest it before undergoing X-rays of the digestive tract. However, technical-grade barium sulfate may contain a dangerous impurity: barium sulfide. There have been fatal incidents when patients mistakenly consumed technical-grade barium sulfate instead of the purified medical form. Therefore, technical barium sulfate should always be treated as a toxic substance: avoid inhaling its dust and prevent it from entering the body. |

Combustion of Thermite: Barium Sulfate/Aluminum (BaSO4/Al) |

|

|

|

|

|

|

|

|

|

|

|
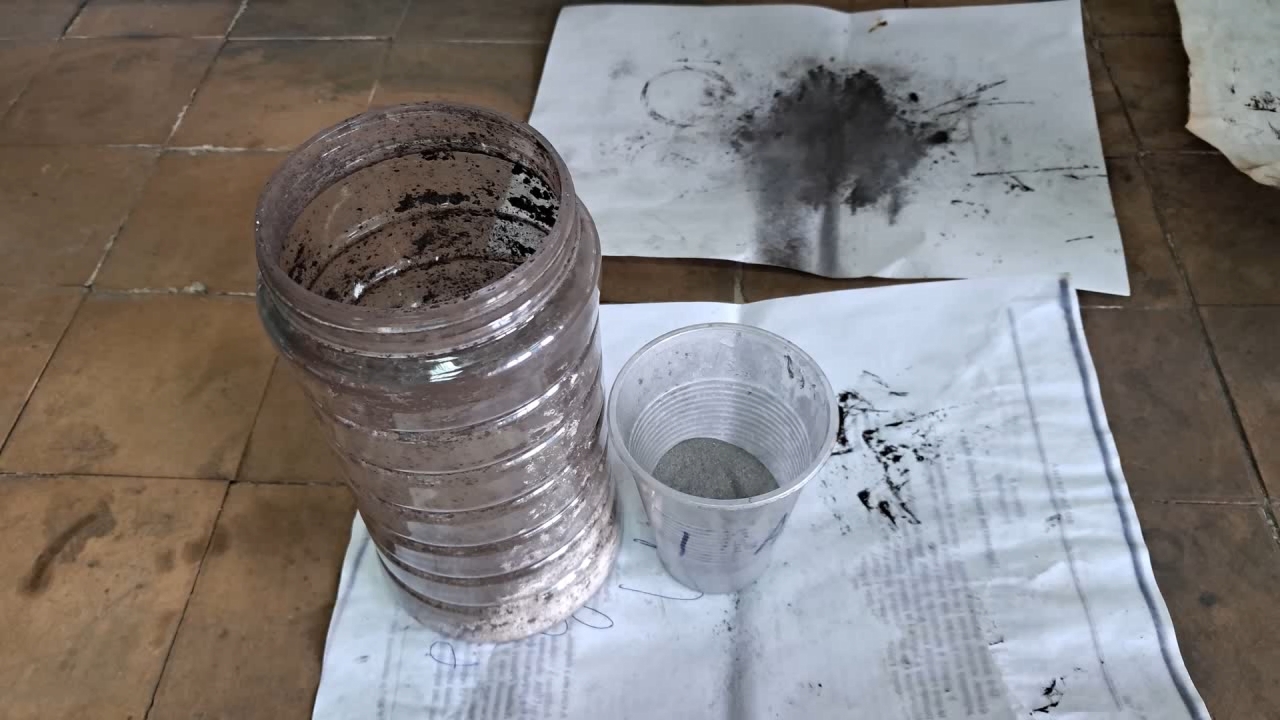
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
Having noticed a mistake in the text, allocate it and press Ctrl-Enter
Combustion of Thermite: Iron(II, III) Oxide, Barium Sulfate, Aluminum (Fe3O4/BaSO4/Al) - Part 29
In the previous part of the article, I described the combustion of BaSO4/Al thermite. I have no doubt that this reaction has been thoroughly studied and documented in the literature. However, I chose not to conduct an unnecessary literature review, so I did not know how the mixture would behave at the time of the experiment.
Горение термита: оксид железа (II, III), сульфат бария, алюминий (Fe3O4/BaSO4/Al) - часть 29 Nevertheless, I found the following composition in the pyrotechnics textbook (by weight %): * Al - 25.3 * FexOy - 59.2 * BaSO4 - 15.3 This is a modified aluminum/iron oxide thermite in which part of the iron oxide has been replaced with barium sulfate—presumably to enhance its ability to burn through objects. Based on these proportions, I weighed out 50.6 g of aluminum, 118.4 g of iron(II,III) oxide, and 30.6 g of barium sulfate. I mixed the substances thoroughly and pressed the mixture into a cut aluminum beer can using hammer blows. I made a depression in the thermite and placed an incendiary mixture consisting of 1.65 g of finely dispersed aluminum and 3.00 g of iron oxide (Fe3O4) into it. Since the previous experiment encountered ignition difficulties with the incendiary mixture, this time I increased the aluminum content and mixed the components more thoroughly. I placed the can containing the thermite on an iron sheet and positioned another one underneath it—the same sheet used in the previous experiment, which had already been burned through. The experiment was conducted outdoors. I aimed a strong flame from a gas burner at the incendiary mixture. This time, it ignited quickly, triggering the combustion of the main thermite charge. A blinding white flame appeared, accompanied by heavy smoke and a shower of sparks. The reaction produced a loud hissing sound. A "lake" of molten material formed on the iron sheet, and a "yellow stream" of molten iron flowed out. Both sheets of iron were burned through. As in the previous test, the flame appears yellow in the video due to the camera's inaccurate color reproduction. Incidentally, during the previous experiment involving the barium sulfate and aluminum mixture, a colleague claimed the flame had a green tint. I did not observe any green hue in either that test or the current one. Most likely, my colleague saw a green tint because he expected to see one. In pyrotechnics, barium salts such as chloride, nitrate, carbonate, chlorate, and perchlorate are commonly used to produce green or blue flames. This time, the same colleague also filmed the experiment using another smartphone. This served as a backup in case I encountered any filming issues. |

Combustion of Thermite: Iron(II, III) Oxide, Barium Sulfate, Aluminum (Fe3O4/BaSO4/Al) |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
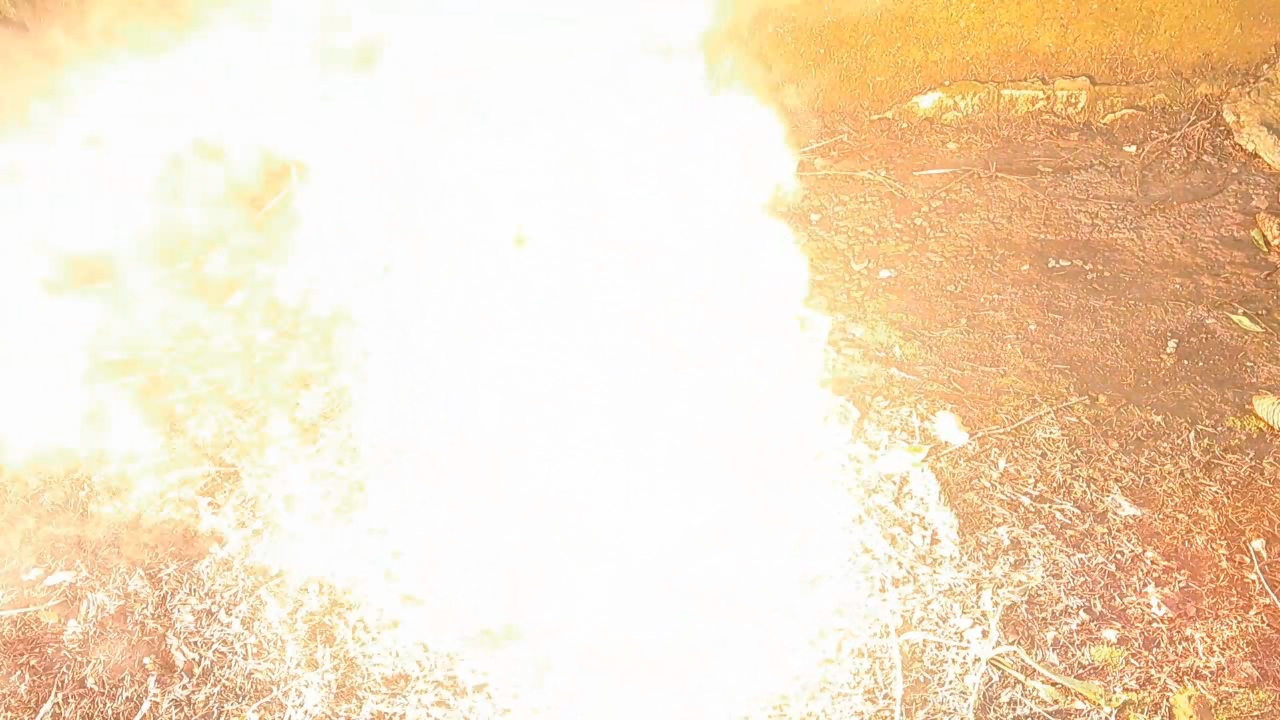
|

|

|
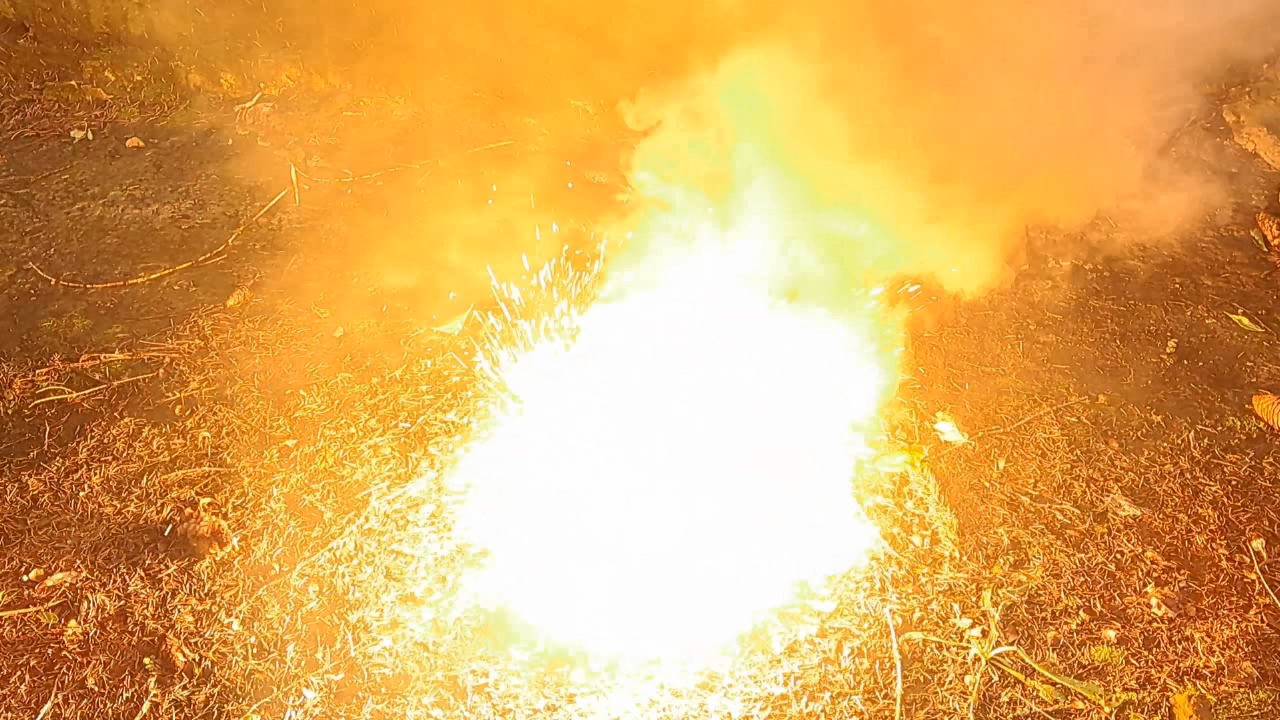
|

|

|

|

|

|

|

|

|

|
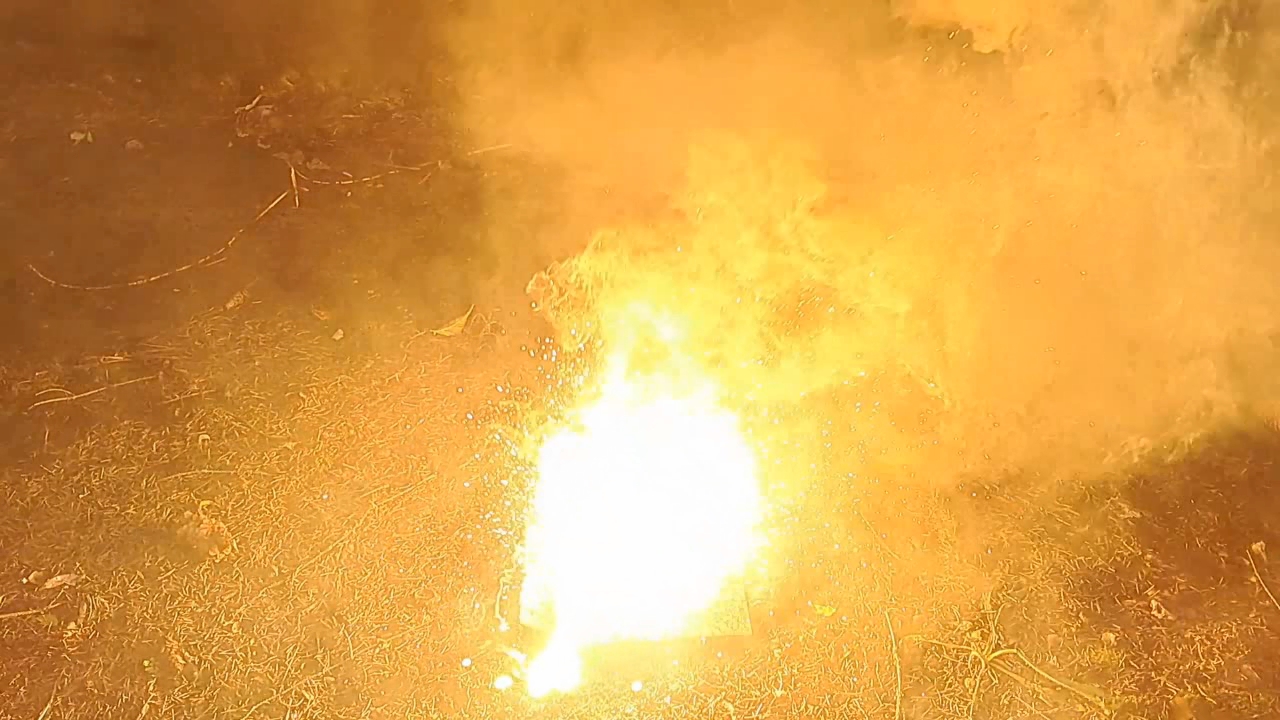
|
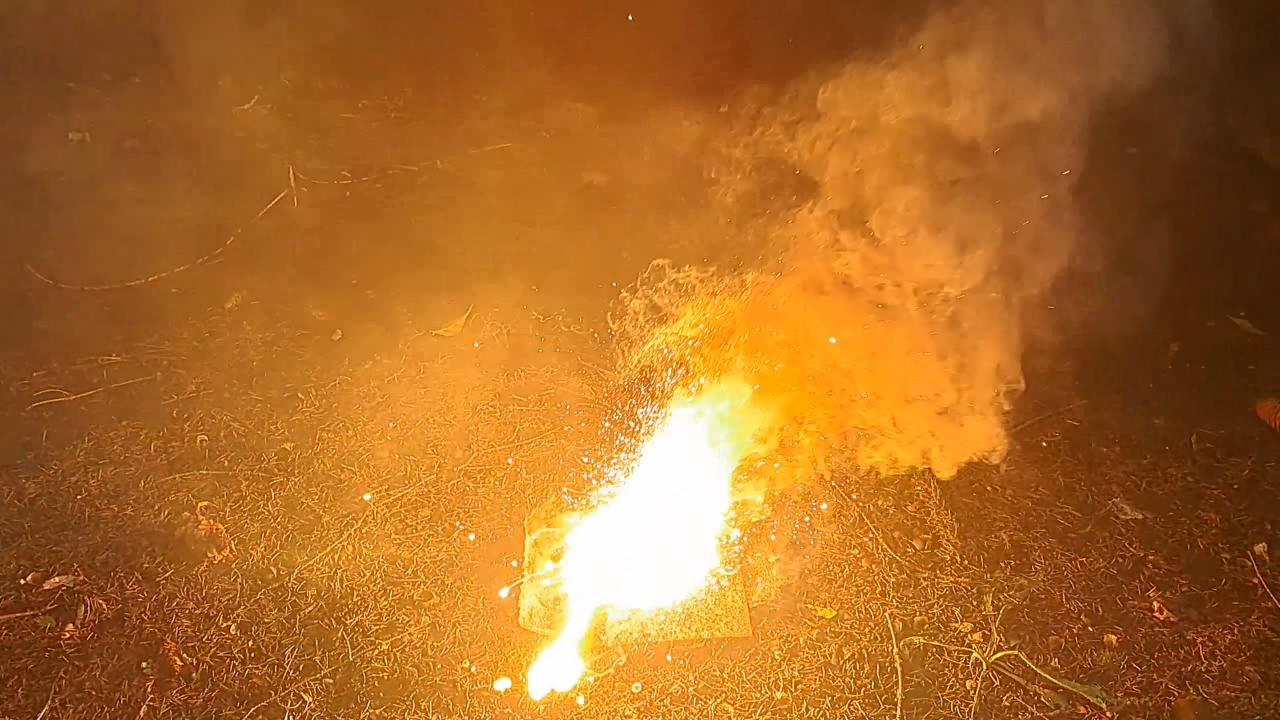
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|