Chemistry and Chemists № 2 2025
Journal of Chemists-Enthusiasts
| Content | Chemistry experiments - video | Physics experiments - video | Home Page - Chemistry and Chemists |
|
Chemistry and Chemists № 2 2025 Journal of Chemists-Enthusiasts |
Experiments with Thermite - pt.3, 4 Chemist |
|
Having noticed a mistake in the text, allocate it and press Ctrl-Enter
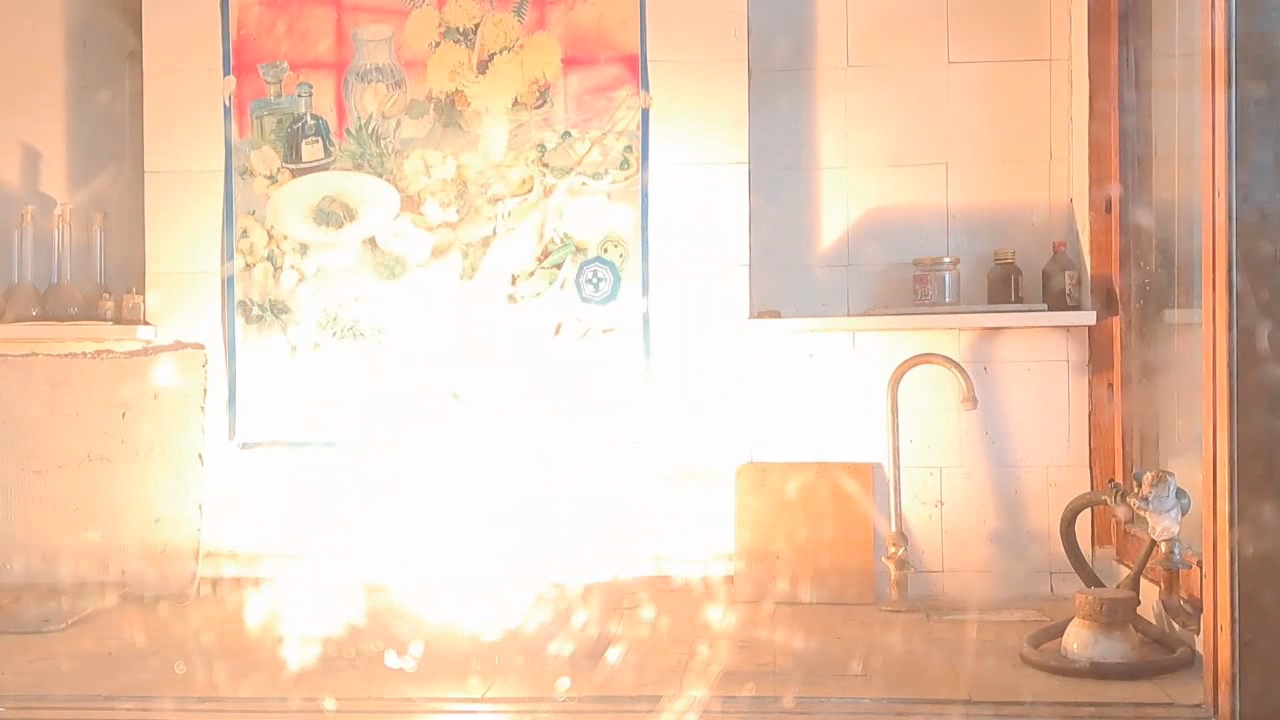
Combustion of Thermite: Iron(II, III) Oxide/Aluminum (Fe3O4/Al) - Part 3
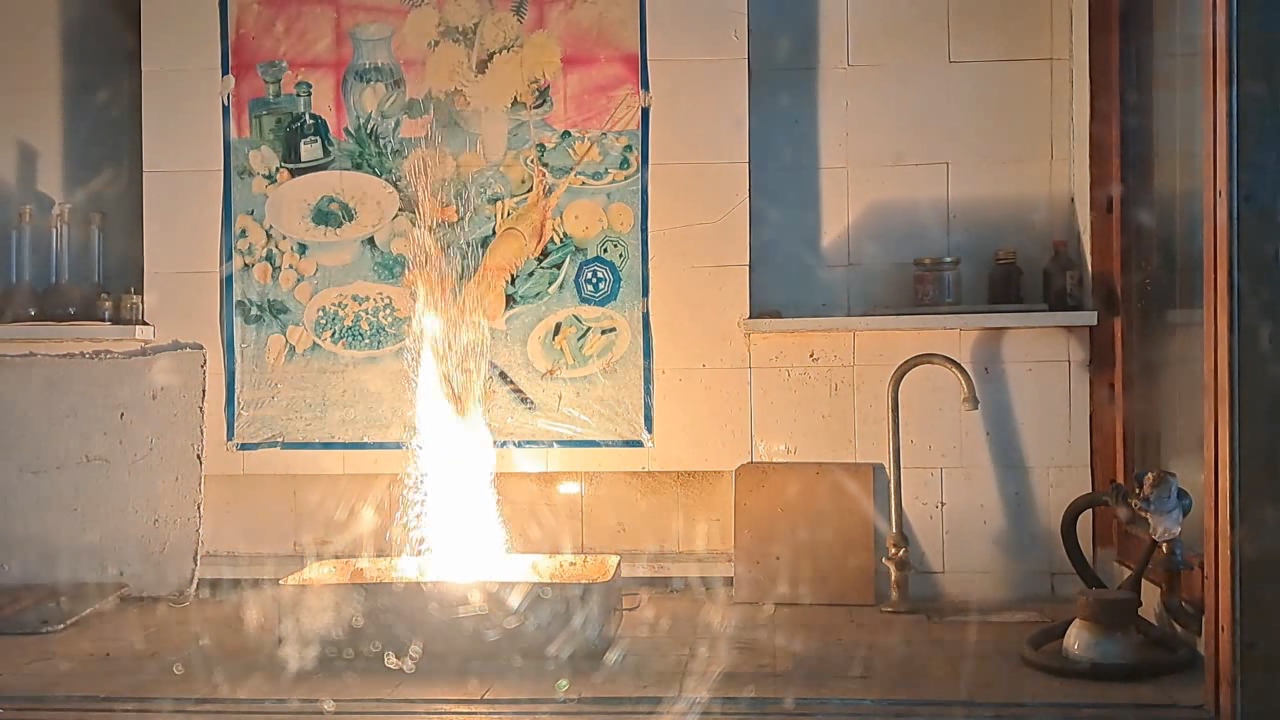
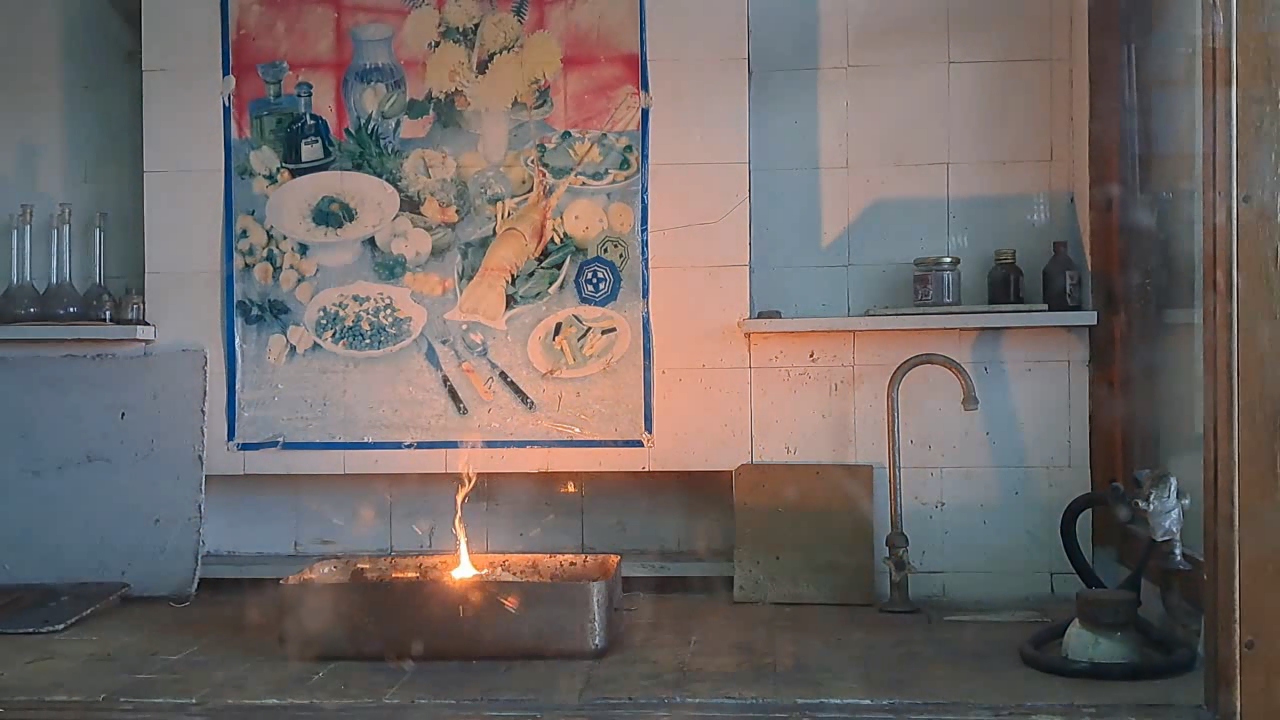
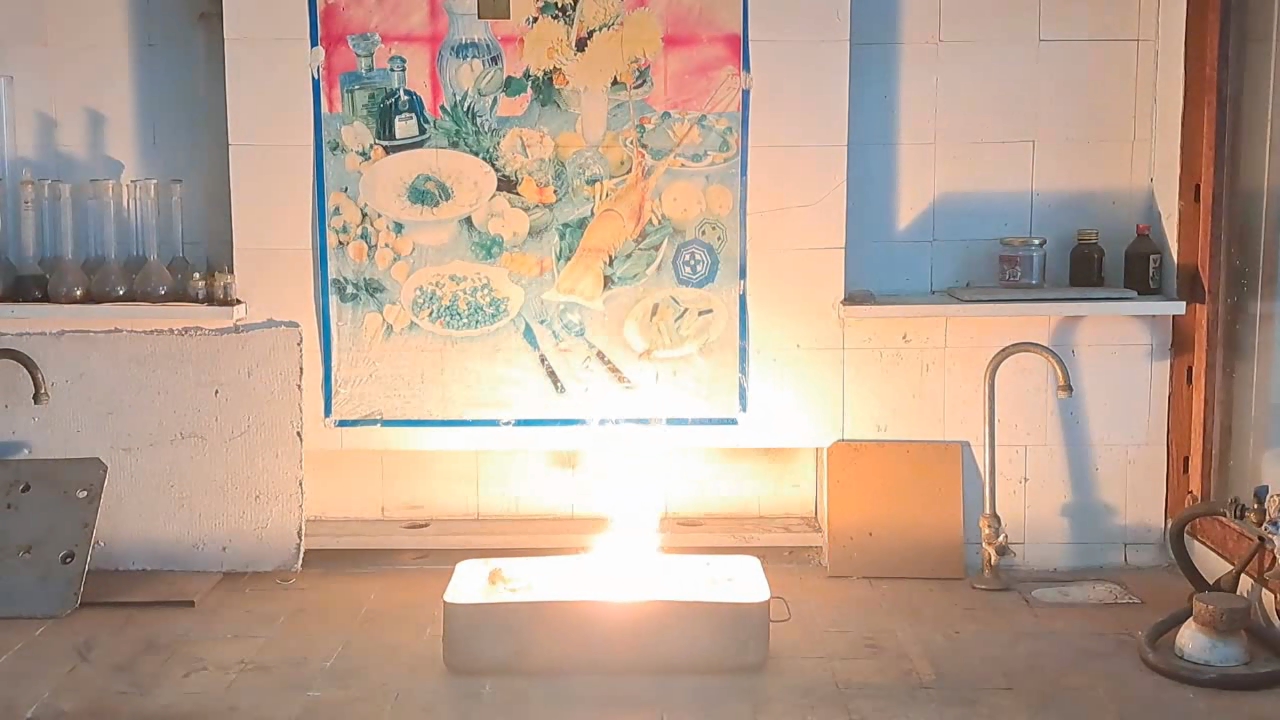
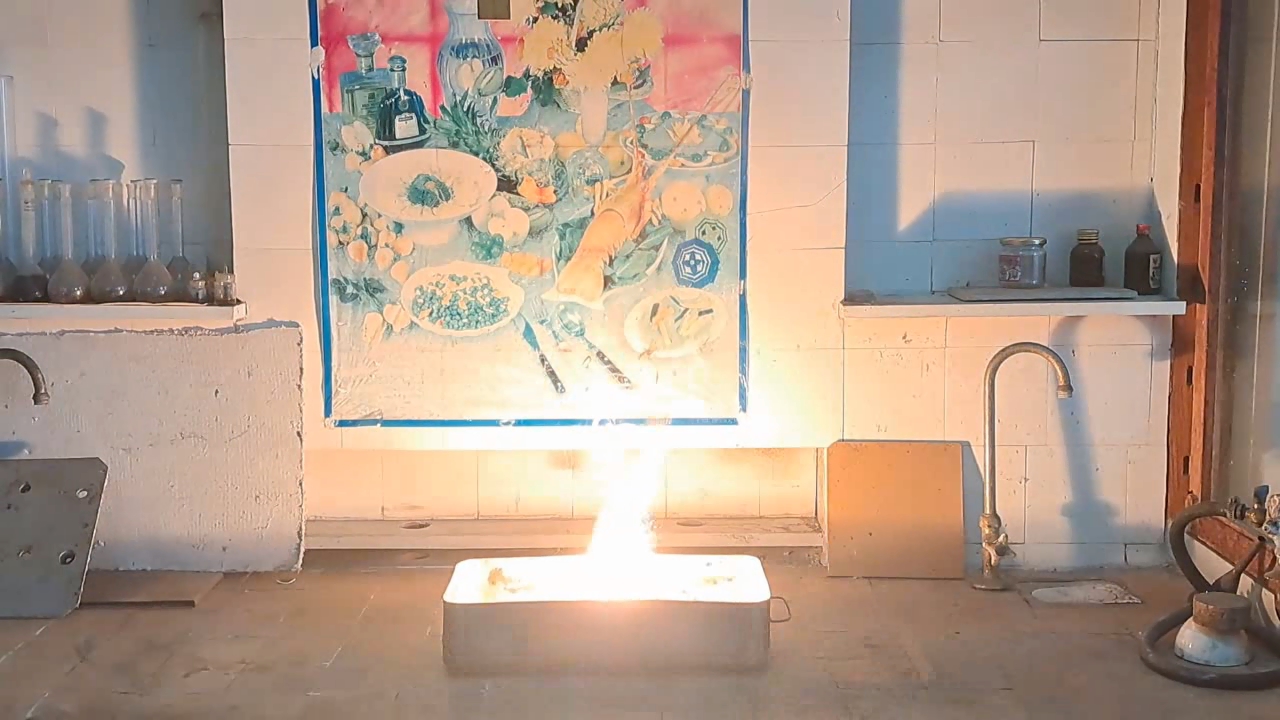
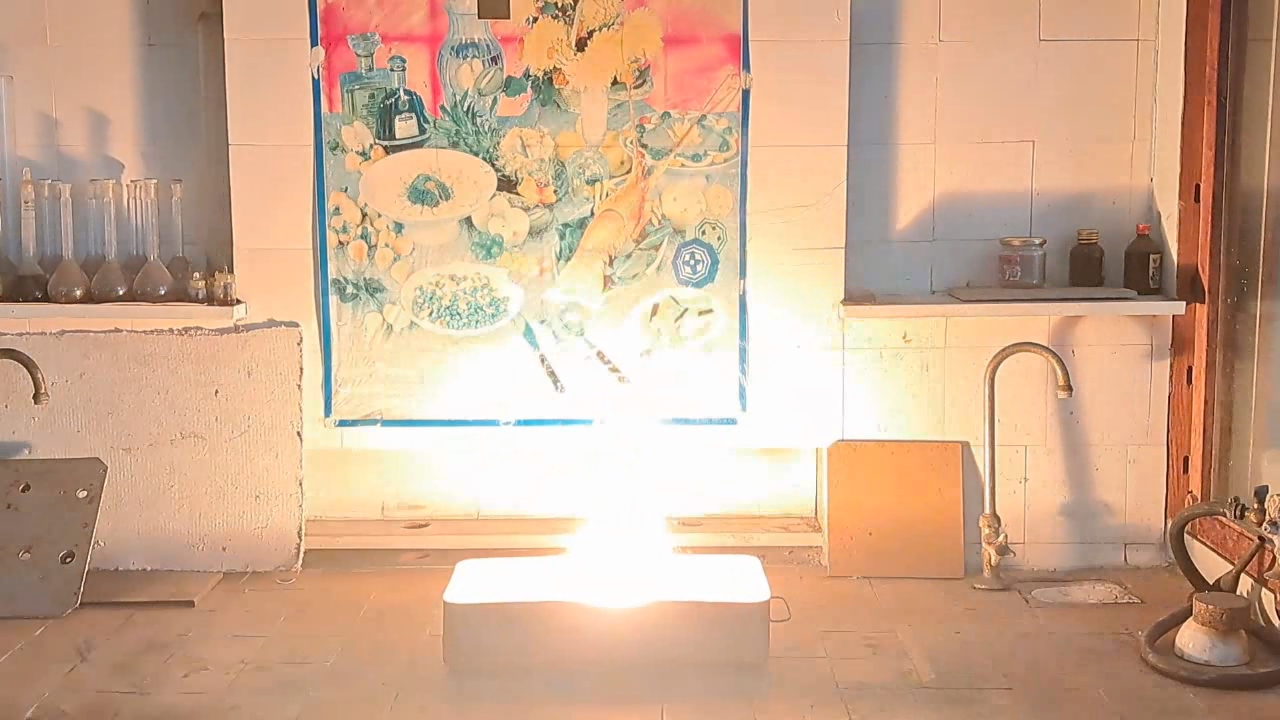
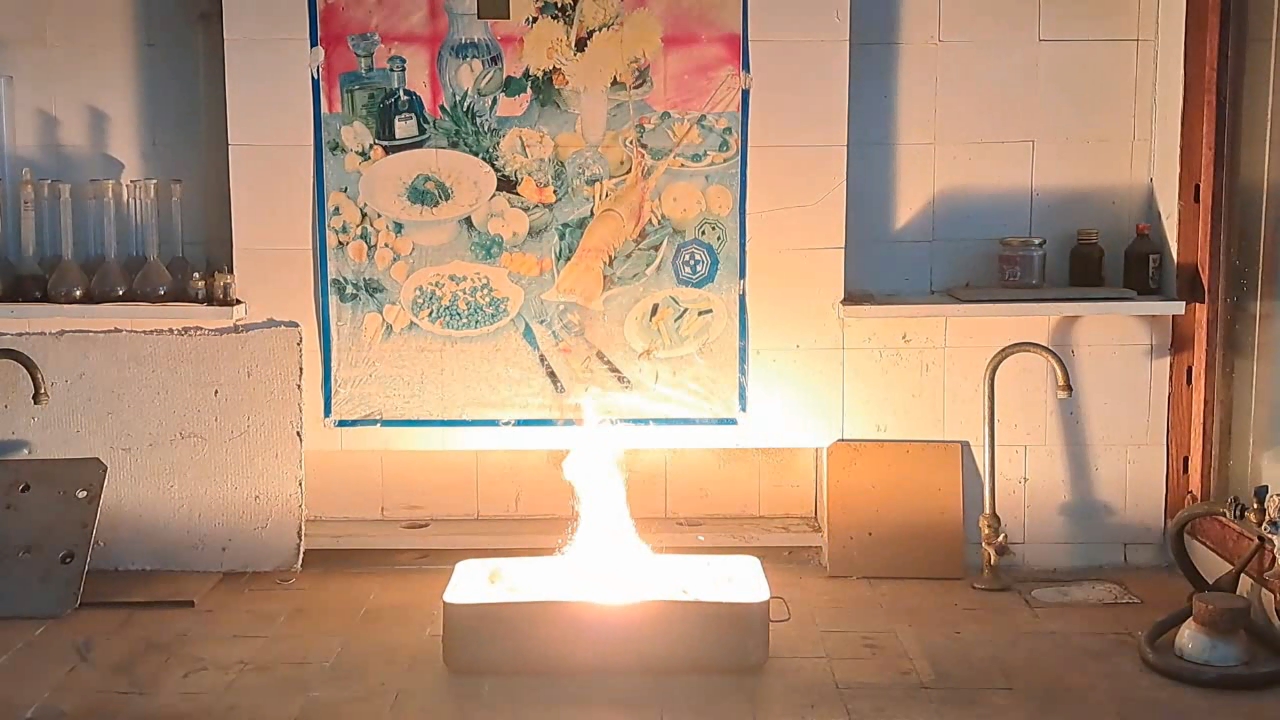
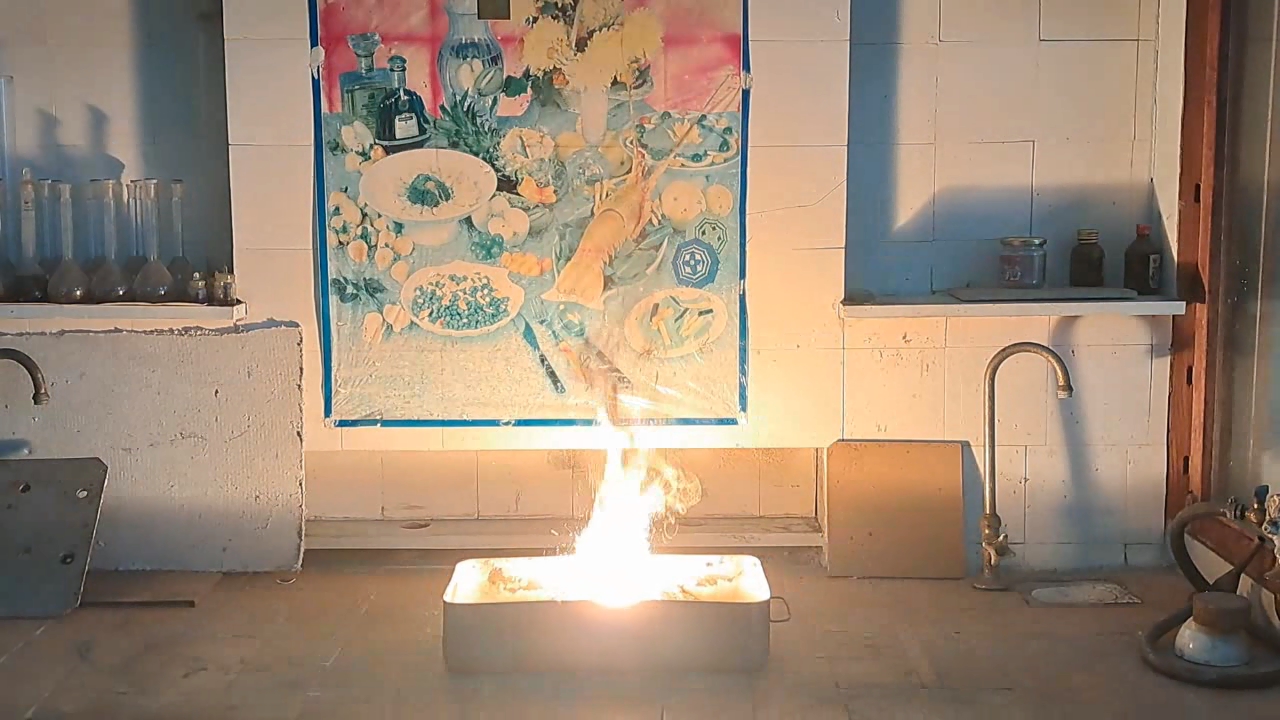
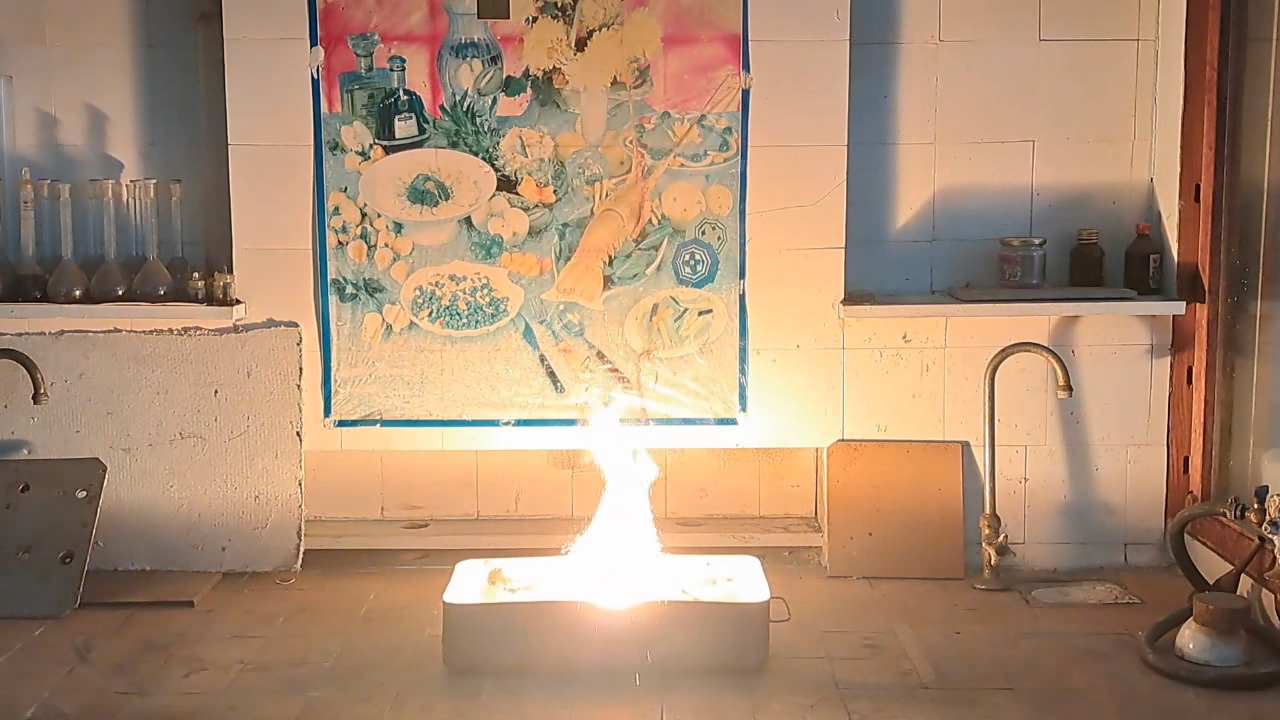
For the third experiment, I used 3 g of finely dispersed aluminum and 8 g of iron(II, III) oxide (Fe3O4). I mixed the powders and placed them in an aluminum foil tube, which I then set in an iron tray filled with dry sand. I invited a colleague to watch the thermite reaction - the same colleague who had previously advised me to use a porcelain crucible for the thermite, which resulted in the crucible shattering and splashing molten iron.
Горение термита: оксид железа (II, III)/алюминий (Al/Fe3O4) - часть 3 As before, I set up a protective plexiglass screen in front of my smartphone. I ignited the thermite using a gas burner. The mixture caught fire with a yellow flame, scattering beautiful sparks. The reaction lasted about six seconds, producing an irregularly shaped piece of iron. During the experiment, an unfortunate incident occurred. As soon as I ignited the thermite, I looked away to avoid being blinded by the flame. When I glanced at the camera, I saw that my colleague was about to remove my smartphone from the tripod to film while holding it. Touching the camera during a dynamic experiment is a bad idea, as it can ruin the footage. I gestured for him to stop, which he did, but in the process, he moved too close to the plexiglass screen, causing his reflection to appear in the video. Additionally, the screen significantly distorted the footage. To obtain a higher-quality recording, I removed the plexiglass screen and repeated the experiment. The thermite burned in a similar manner, but the video turned out much better. |

Combustion of Thermite: Iron(II, III) Oxide/Aluminum (Fe3O4/Al) |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
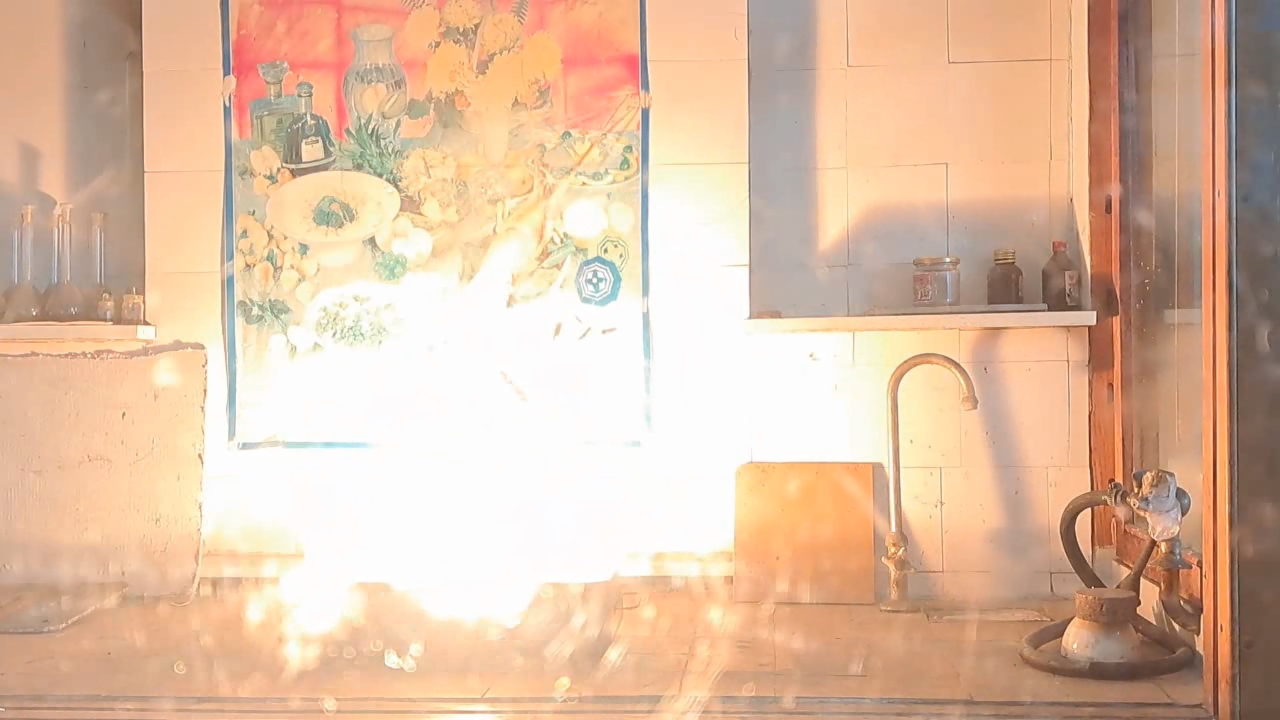
|
|
|

|
|

|

|
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
|
|
|
|

|
|
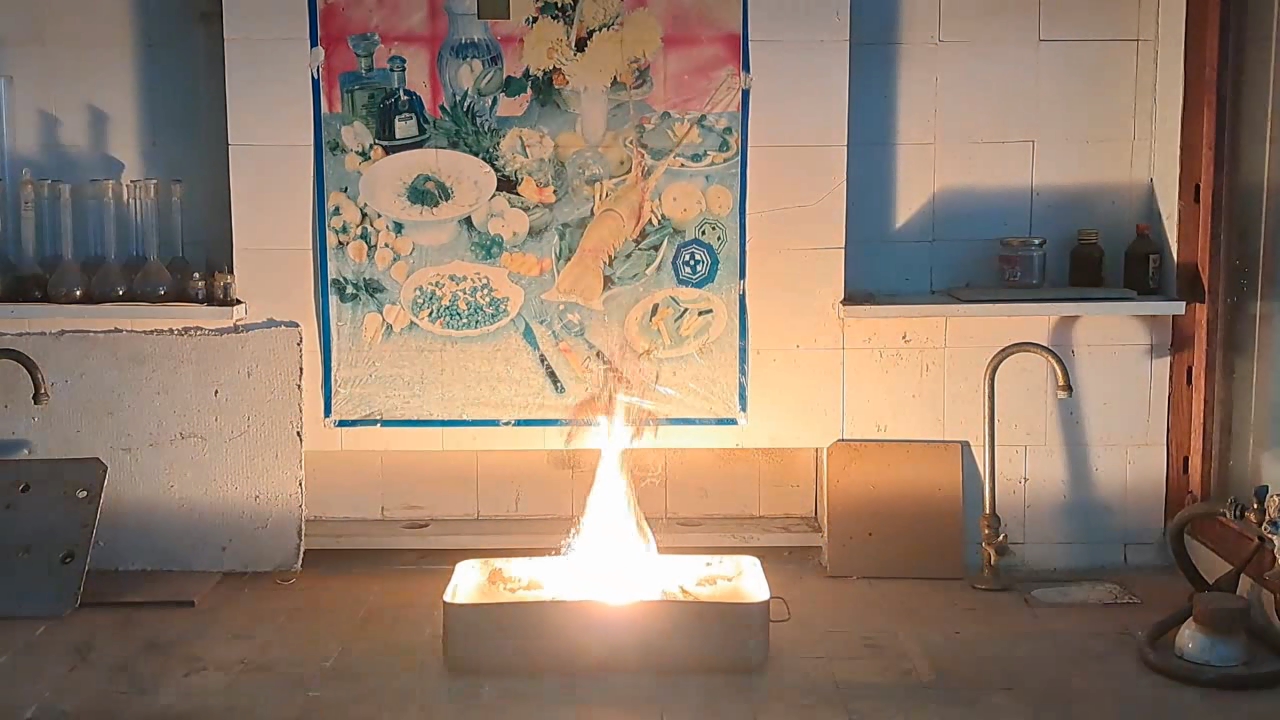
|
|
|
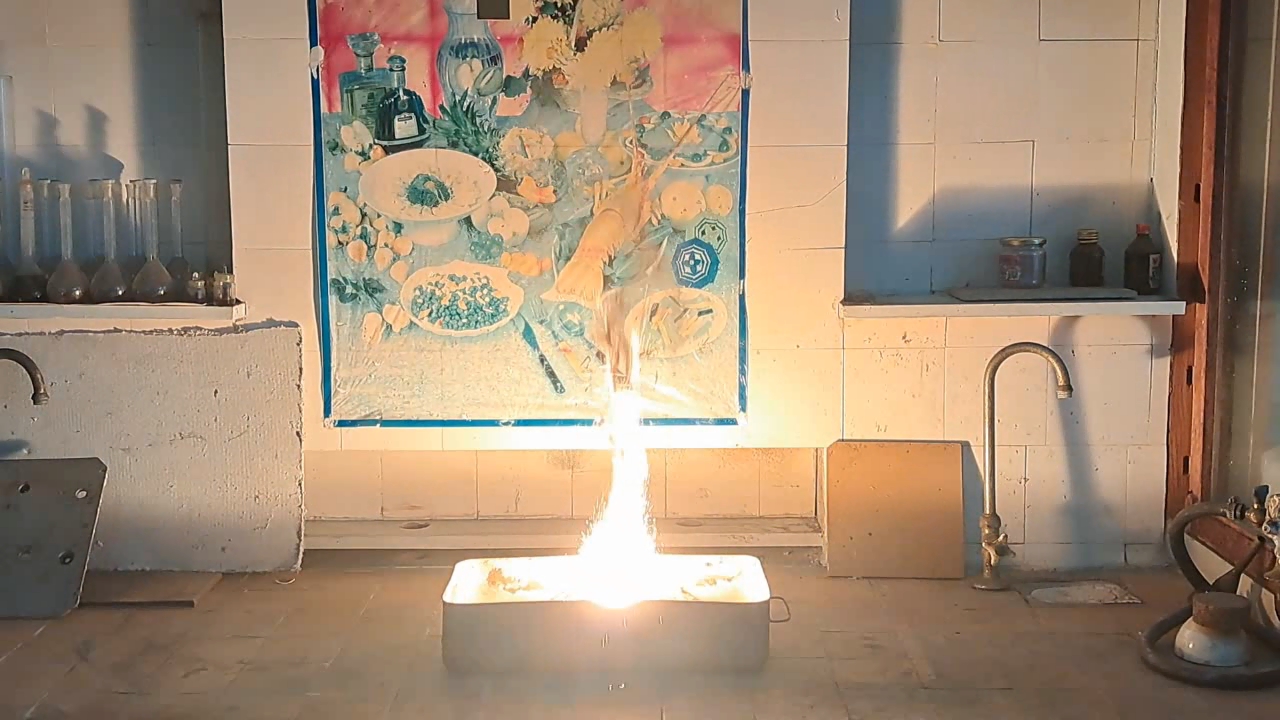
|
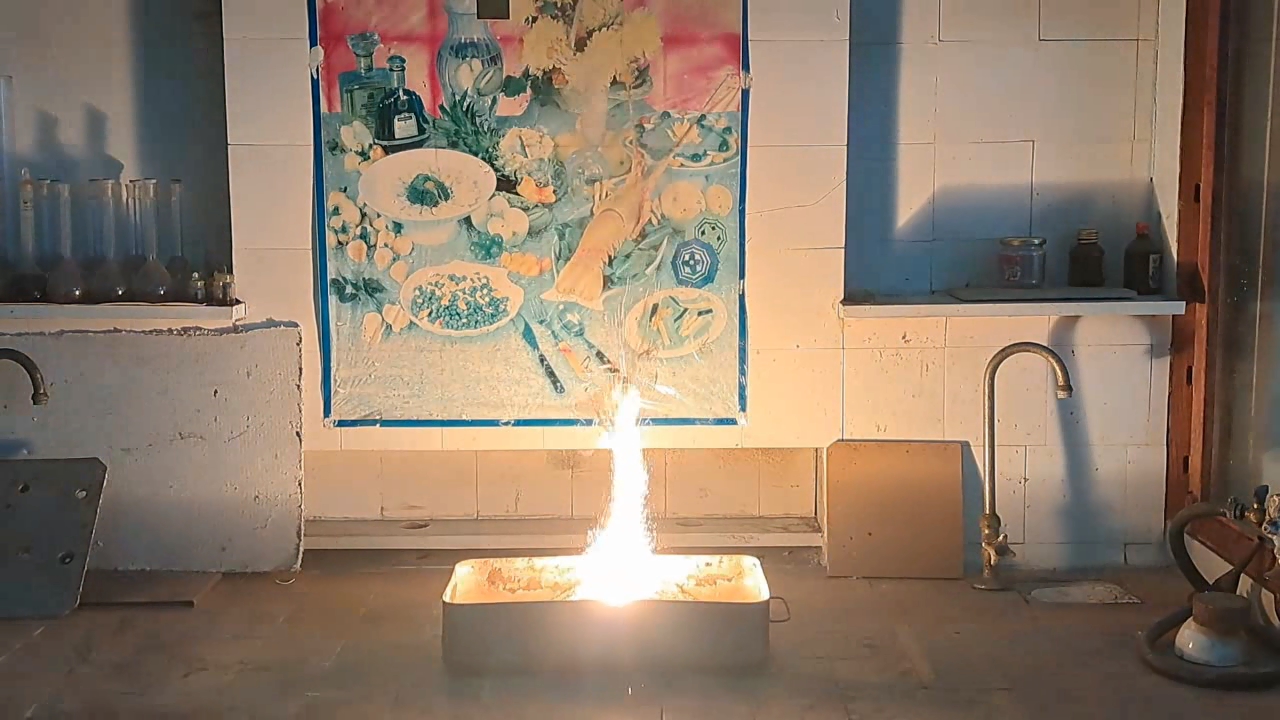
|

|

|

|

|

|

|
|
Having noticed a mistake in the text, allocate it and press Ctrl-Enter
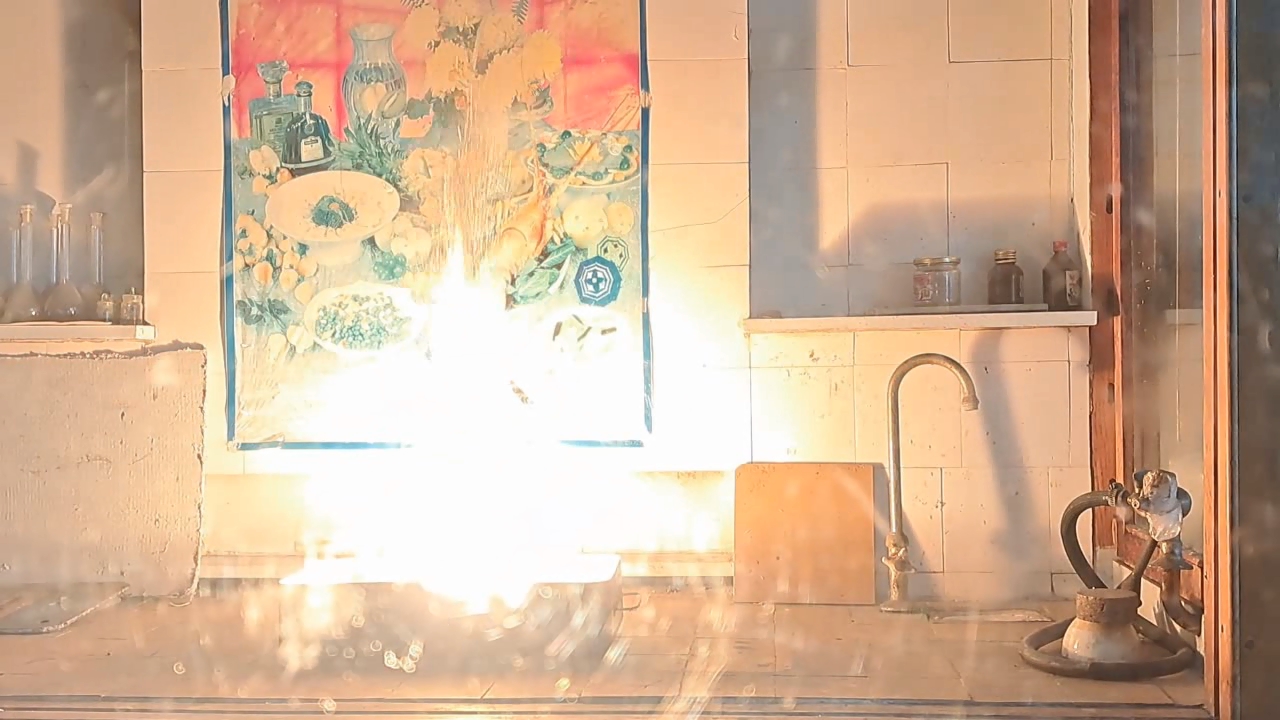
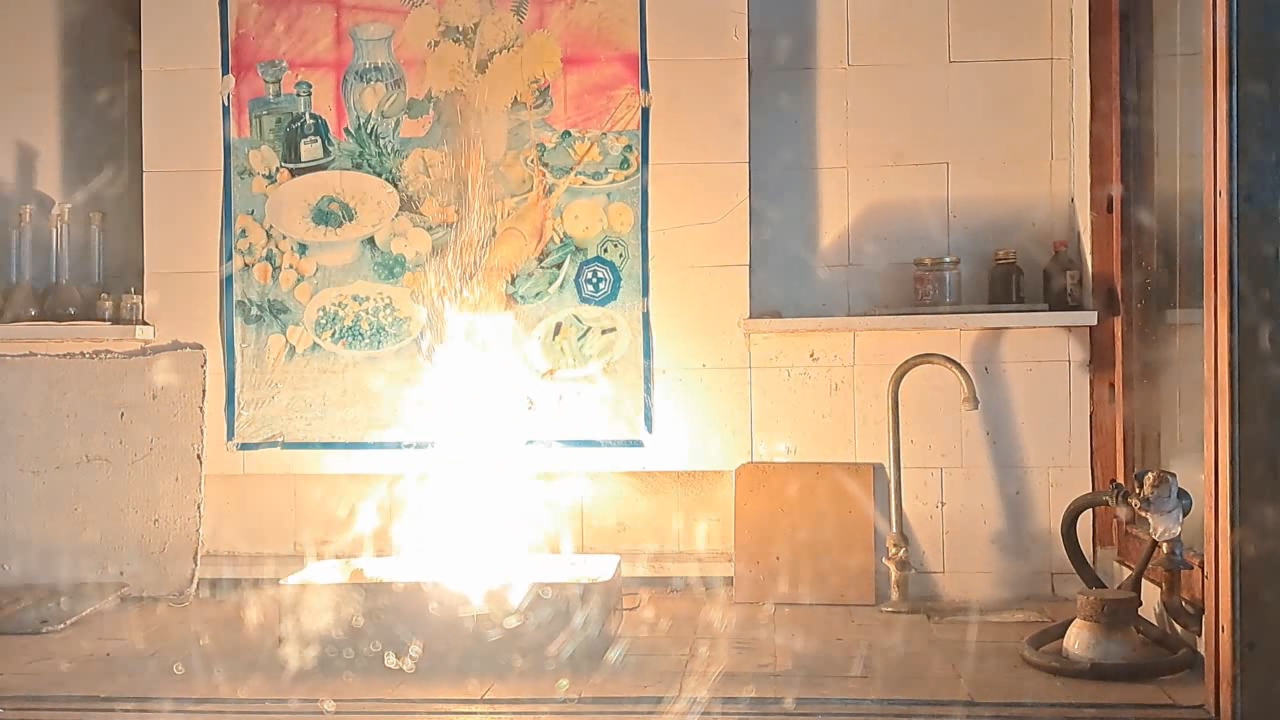
Combustion of Thermite: Iron(II, III) Oxide/Aluminum (Waste), Fe3O4/Al - Part 4
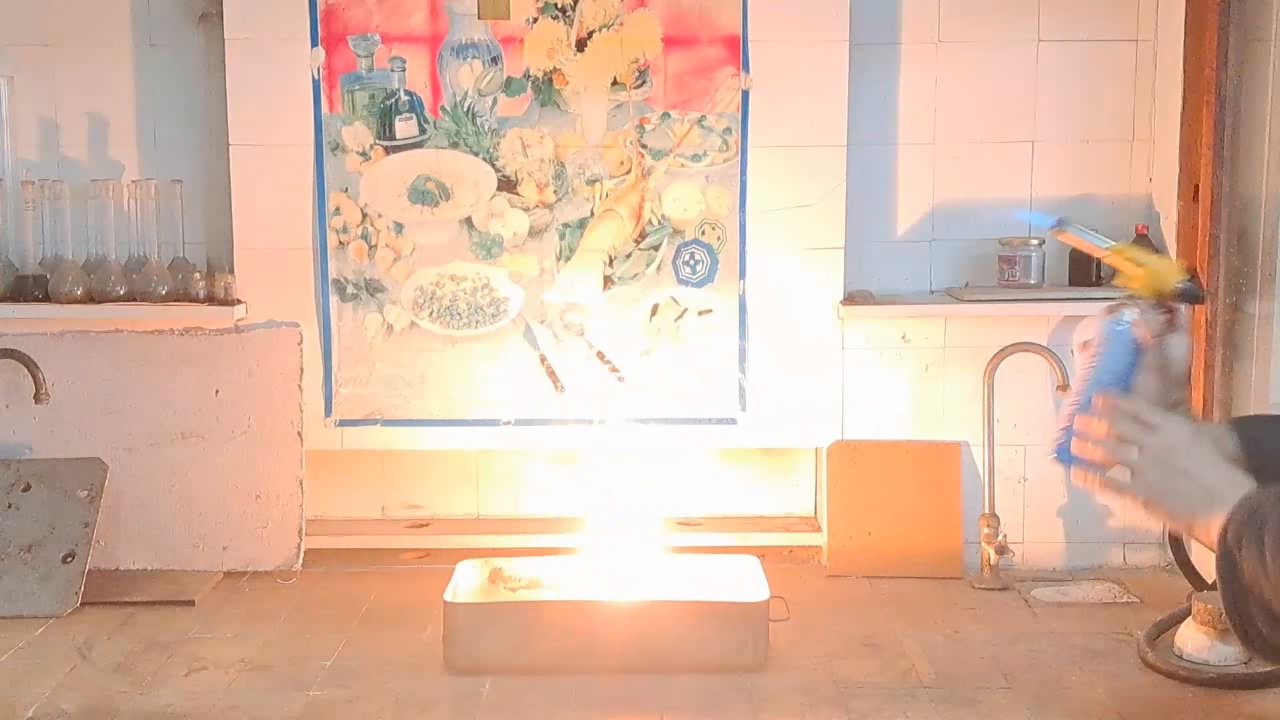
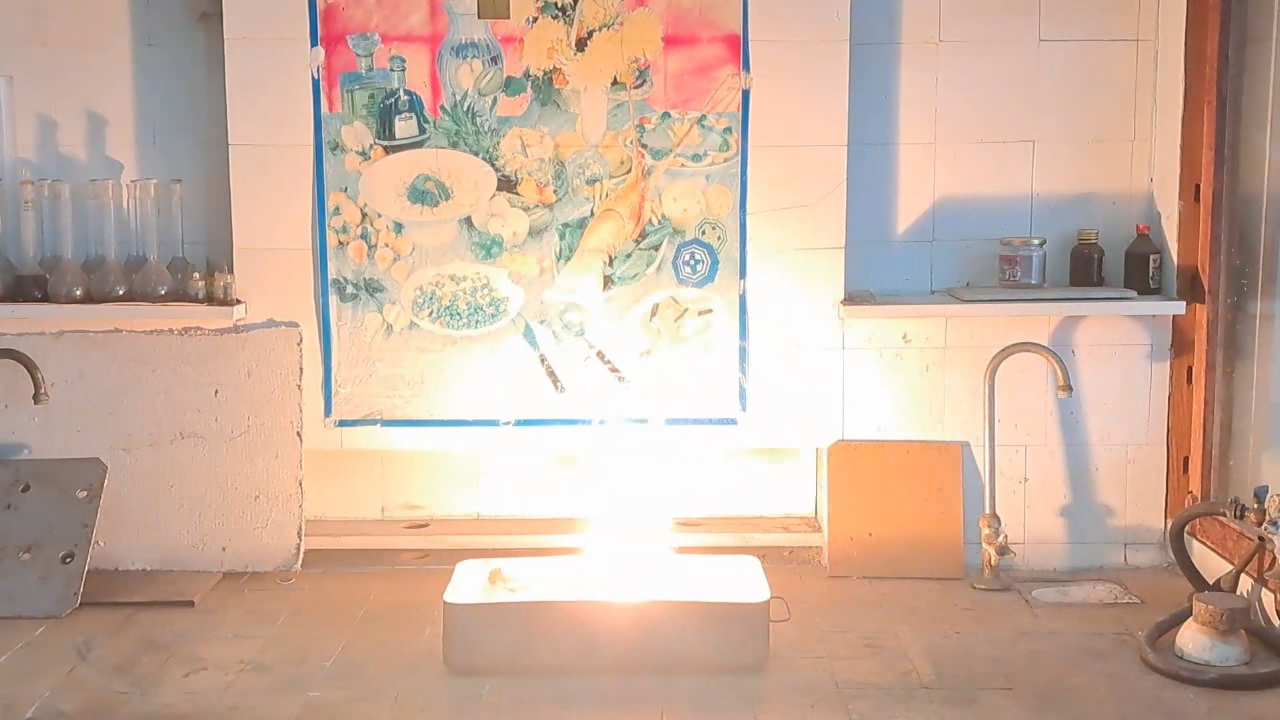
After conducting several comparison experiments using high-quality aluminum, I moved on to testing aluminum waste obtained from aluminum wire production. The waste was a dark gray mass in which aluminum fibers were distinguishable.
Горение термита: оксид железа (II, III)/алюминий (отходы), Al/Fe3O4 - часть 4 Similar to the previous experiment, I weighed 3 grams of aluminum and 8 grams of iron oxide (Fe3O4), then mixed the substances in an iron mortar. Initially, the waste was wet, but after being stored in a loosely sealed jar, it dried out. However, I was unsure whether all the moisture had evaporated completely, so I further dried the prepared thermite in a drying cabinet at 95°C. The presence of water in the thermite could cause the spattering of hot particles during combustion. After two hours of drying, I placed the thermite in an aluminum foil tube and compacted it. I then positioned the tube in sand inside an iron tray and directed a strong gas burner flame at the thermite. Unlike in previous experiments, igniting this thermite proved to be quite difficult. Sparks appeared, and the upper layer of the mixture glowed yellow-hot, but the thermite refused to ignite. Several times, the mixture briefly flared up but quickly died out—the combustion did not propagate deeply into the thermite. After a minute of unsuccessful attempts, the thermite finally ignited, but the reaction was less vigorous than that of thermite made with high-quality aluminum. Moreover, the lower part of the tube, which was immersed in sand, did not burn at all. This thermite is poorly suited for practical applications. I recalled that thermite composed solely of aluminum powder and iron oxides is rarely used as an incendiary composition in military applications. To enhance combustion and facilitate ignition, a portion of the iron oxides is typically replaced with more reactive oxidizers. Additionally, binders and various additives are introduced into such thermites. So, I need to consult military textbooks and adapt the compositions they provide, replacing expensive and scarce oxidizers with affordable and widely available ones—such as potassium nitrate. |

Combustion of Thermite: Iron(II, III) Oxide/Aluminum (Waste), Fe3O4/Al |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|