Chemistry and Chemists № 2 2025
Journal of Chemists-Enthusiasts
| Content | Chemistry experiments - video | Physics experiments - video | Home Page - Chemistry and Chemists |
|
Chemistry and Chemists № 2 2025 Journal of Chemists-Enthusiasts |
Experiments with Thermite - pt.5, 6, 7 Chemist |
|
Having noticed a mistake in the text, allocate it and press Ctrl-Enter
Combustion of Thermite: Iron(II, III) Oxide/Aluminum (Fe3O4/Al) - Part 5
In a pyrotechnics textbook, I found the following basic thermite composition:
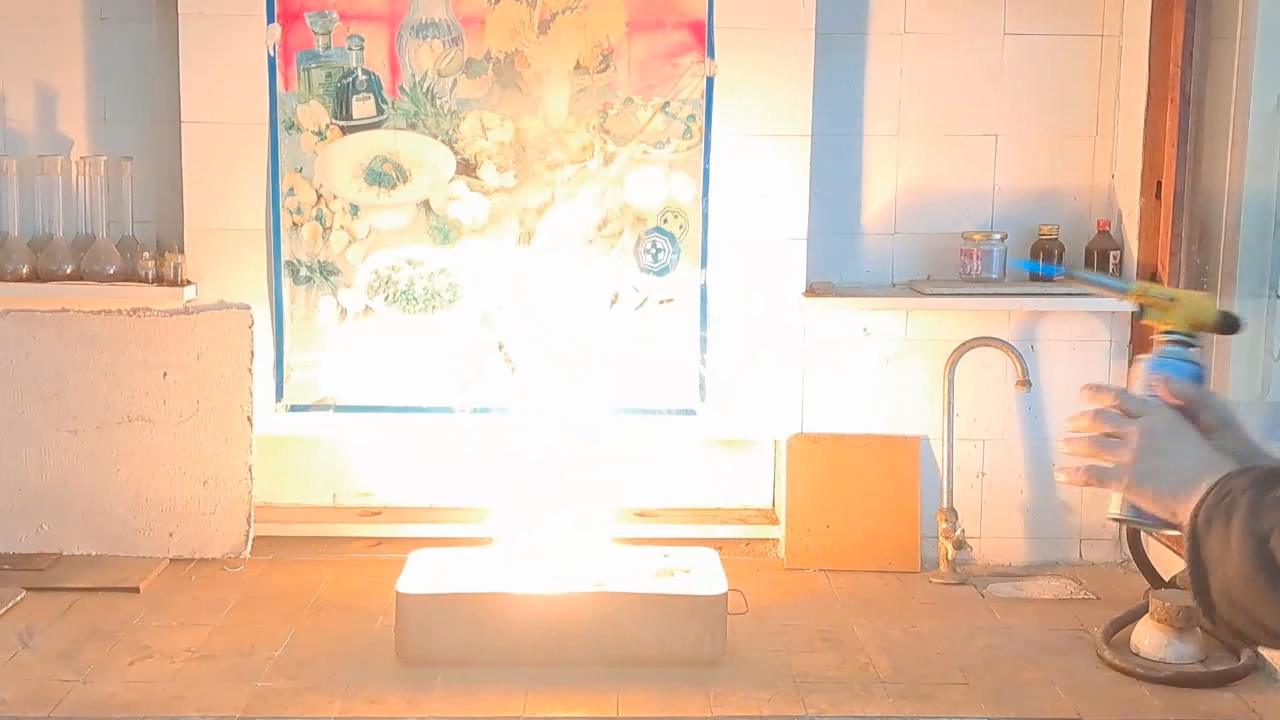
Горение термита: оксид железа (II, III)/алюминий (Al/Fe3O4) - часть 5 Fe3O4 - 2.75; Al - 1 (by weight). This composition is close to the one I used in previous experiments (the difference is minor). The current objective is to prepare thermite using aluminum waste. I planned to approach this task in three stages. First, test the basic composition by increasing the quantity of the components: 5 g of aluminum and 13.75 g of iron oxide (Fe3O4). For this experiment, it was necessary to use high-quality, finely dispersed aluminum to prepare the thermite. As described in the previous part of the article, thermite made from iron oxide (Fe3O4) and aluminum waste ignited and burned very poorly. Repeating this experiment with a simple increase in the amount of aluminum waste seemed illogical. It was necessary to select an improved thermite composition from the table provided in the book, replacing the additional oxidizers with a cheaper and more accessible alternative - potassium nitrate. Then, this new composition had to be prepared and tested using high-quality, finely dispersed aluminum.  Finally, the experiment had to be repeated using aluminum waste instead of high-quality aluminum in the improved composition. Stage One: I weighed the components (5 g of aluminum and 13.5 g of iron oxide) and prepared the mixture in an iron mortar. I then poured the mixture onto a sheet of paper and made a "bag" by folding the paper around the thermite. I compressed the bag in the palm of my hand to compact the mixture. Next, I placed the thermite on sand inside an iron tray and directed a strong flame from a gas burner at it. Sparks flew, and the paper caught fire, but the thermite did not ignite immediately; compacted thermite is much more difficult to ignite. Eventually, a bright yellow flash appeared as the mixture ignited. Combustion lasted about ten seconds, producing numerous yellow sparks. The solidifying iron resembled the crater of an active volcano. After cooling, a piece of irregularly shaped iron had formed. |

Combustion of Thermite: Iron(II, III) Oxide/Aluminum (Fe3O4/Al) |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
Having noticed a mistake in the text, allocate it and press Ctrl-Enter
Combustion of Thermite (Fe3O4 + KNO3 + Al + S) - Part 6
I selected the following thermite composition from the table above (%wt.):
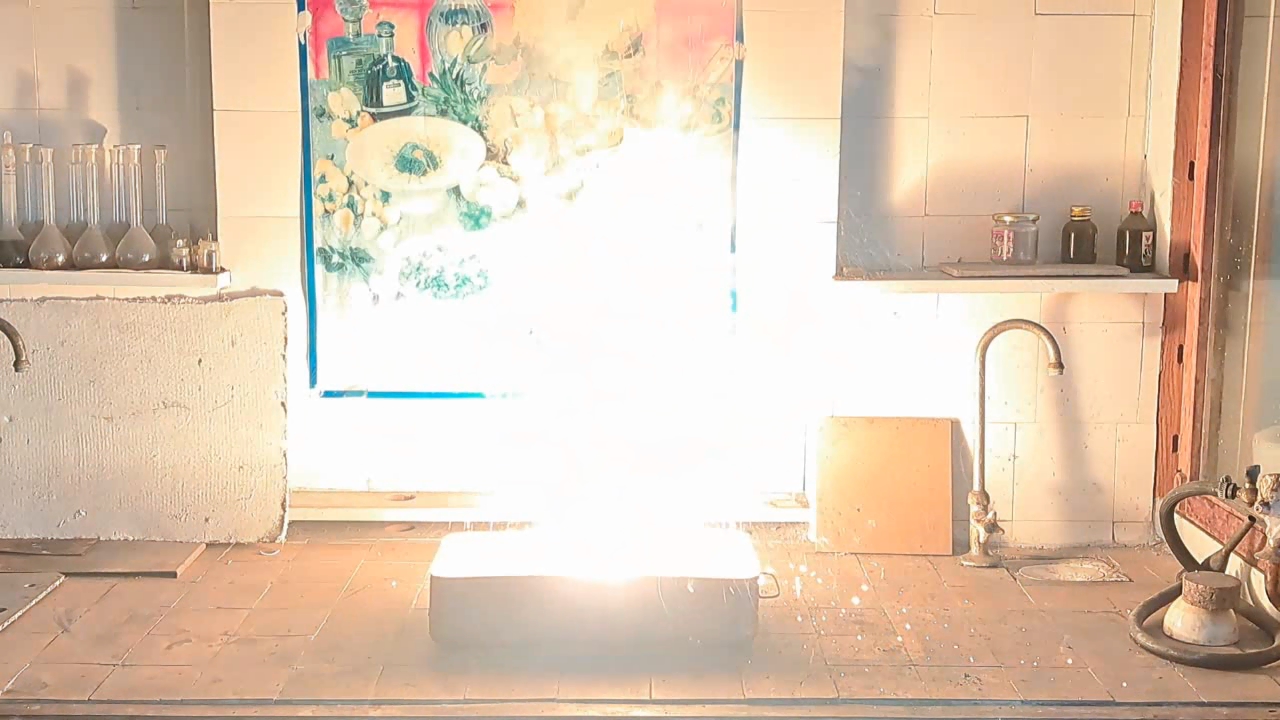
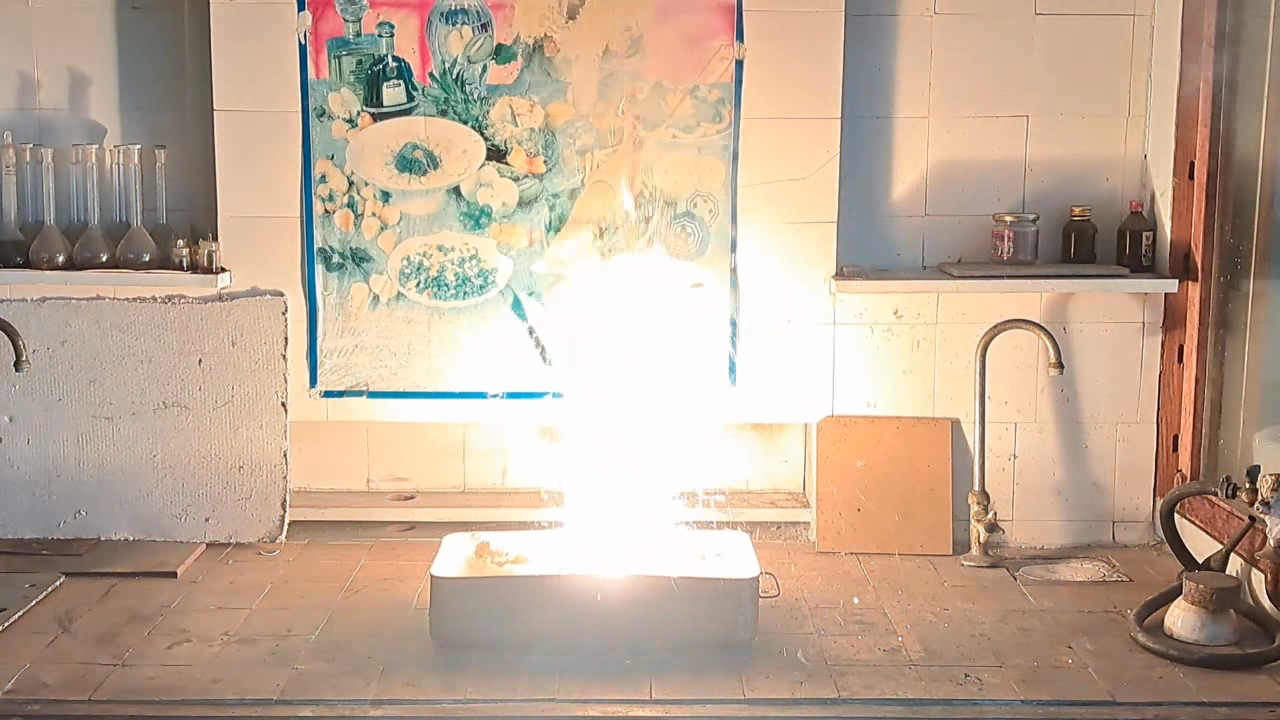
Горение термита (Fe3O4 + KNO3 + Al + S) - часть 6 Iron oxides: 55.2%, aluminum: 25%, barium nitrate: 19.5%, castor oil: 0.3%, and sulfur: 0.9% (over 100%). I recalculated the thermite composition by replacing barium nitrate with an equivalent amount of potassium nitrate and omitting the castor oil (amounts in grams): Iron oxide (Fe3O4): 11.44 g, aluminum: 5.18 g, potassium nitrate: 3.02 g, sulfur: 0.18 g. After the experiment, I discovered a minor error in the calculations: the "sulfur - 0.9%" value originated from a neighboring recipe. Apparently, sulfur in that context was used as a binder. I weighed out the iron oxide, finely dispersed aluminum powder, potassium nitrate, and sulfur. The components were thoroughly mixed. The thermite was compacted by hand, having first been placed in a "bag" made from a sheet of paper. Finally, I set the thermite mixture in a tray filled with sand and ignited it using a strong burner flame. The thermite ignited immediately. The flash was significantly larger and brighter, but shorter in duration compared to the combustion of the Fe3O4 + Al mixture in the previous experiment. After combustion, the formation of a ''drop'' of solidified iron was not observed. Despite the small amount of sulfur, its combustion products produced an unpleasant odor. |

Combustion of Thermite (Fe3O4 + KNO3 + Al + S) |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
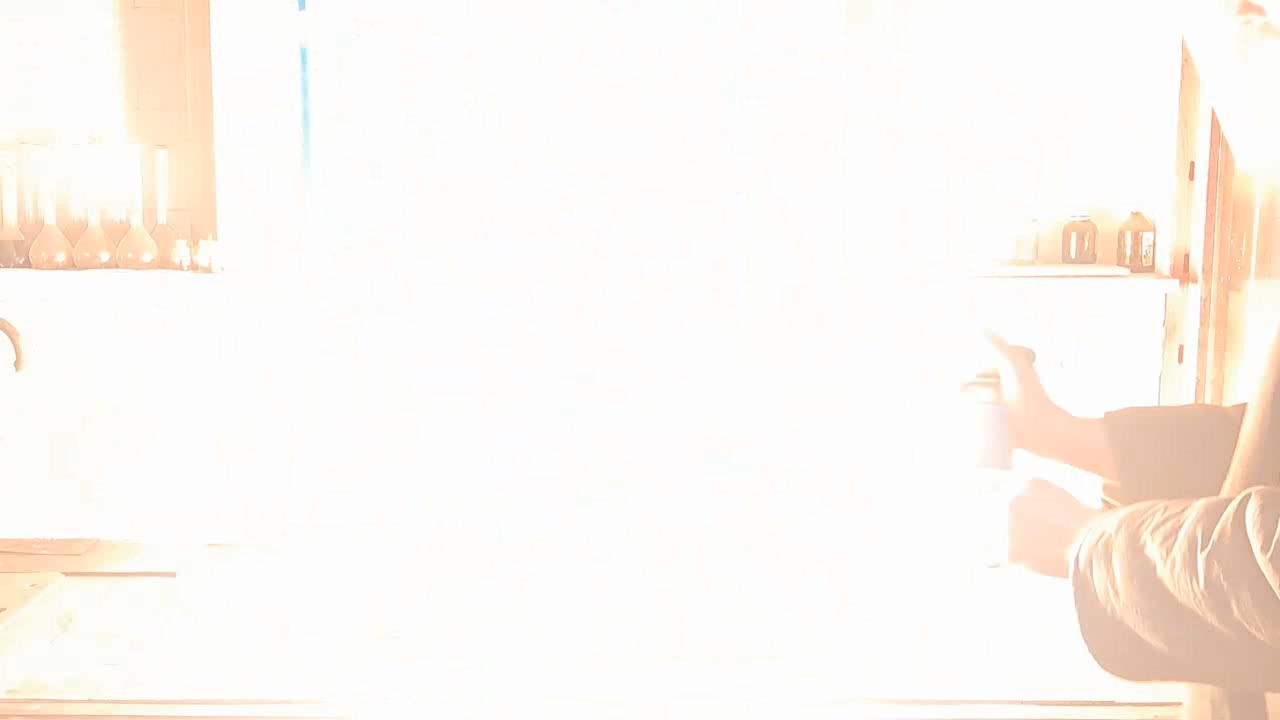
|

|
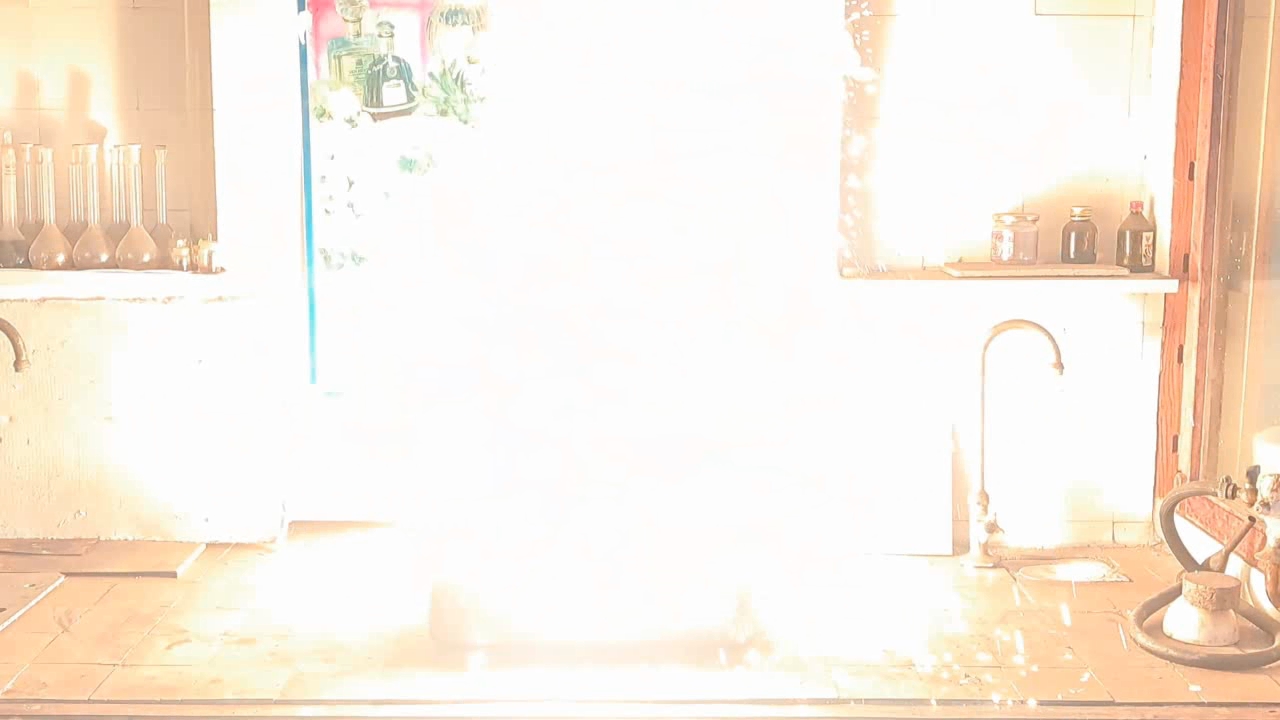
|

|
|
|

|

|

|

|

|

|
|
Having noticed a mistake in the text, allocate it and press Ctrl-Enter
Combustion of Thermite: Fe3O4 + KNO3 + Al (waste) + S - Part 7
I repeated the same procedure as in the previous experiment but replaced the high-quality, finely dispersed aluminum with aluminum waste. I also carefully dried the mixture in a drying cabinet to remove any possible moisture.
Горение термита: Fe3O4 + KNO3 + Al (отходы) + S - часть 7 The thermite ignited with much greater difficulty, burned less vigorously, and took significantly longer to burn than a similar composition made with finely dispersed aluminum. This time, the burning thermite produced an irregularly shaped piece of iron. Thus, it is possible to prepare thermite using aluminum waste, but it burns less efficiently and is more difficult to ignite compared to thermite prepared with high-quality, finely dispersed aluminum |

Combustion of Thermite: Fe3O4 + KNO3 + Al (waste) + S |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|