Chemistry and Chemists № 2 2025
Journal of Chemists-Enthusiasts
| Content | Chemistry experiments - video | Physics experiments - video | Home Page - Chemistry and Chemists |
|
Chemistry and Chemists № 2 2025 Journal of Chemists-Enthusiasts |
Experiments with Thermite - pt.8, 9, 10 Chemist |
|
Having noticed a mistake in the text, allocate it and press Ctrl-Enter
Combustion of Thermite: Fe3O4 + KNO3 + Al (waste) + S - Part 8
I repeated the previous experiment, increasing the amount of thermite mixture prepared from aluminum waste:
Горение термита: Fe3O4 + KNO3 + Al (отходы) + S - часть 8 iron oxide (Fe3O4): 17.2 g, aluminum: 7.8 g, potassium nitrate: 4.5 g, sulfur: 0.3 g. The mixture ignited easily but burned much more slowly than the thermite made with high-quality, finely dispersed aluminum. Additionally, the combustion was uneven. Therefore, I decided to stop experimenting with aluminum waste. |

Combustion of Thermite: Fe3O4 + KNO3 + Al (waste) + S |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
Having noticed a mistake in the text, allocate it and press Ctrl-Enter
Combustion of Thermite: Iron(II, III) Oxide/Aluminum (Fe3O4/Al) - Part 9
As I have already noted, thermite combustion is often demonstrated in a clay flower pot. These pots typically have a hole in the bottom to allow excess water to drain from the soil. When thermite burns, molten iron naturally flows through this hole. I had a clay pot available, but its size made me uneasy: if I filled such a large pot with thermite, I would risk starting a fire in the laboratory. I had only ever seen small-scale thermite reactions and could not predict how a large quantity would behave.
Горение термита: оксид железа (II, III)/алюминий (Al/Fe3O4) - часть 9  
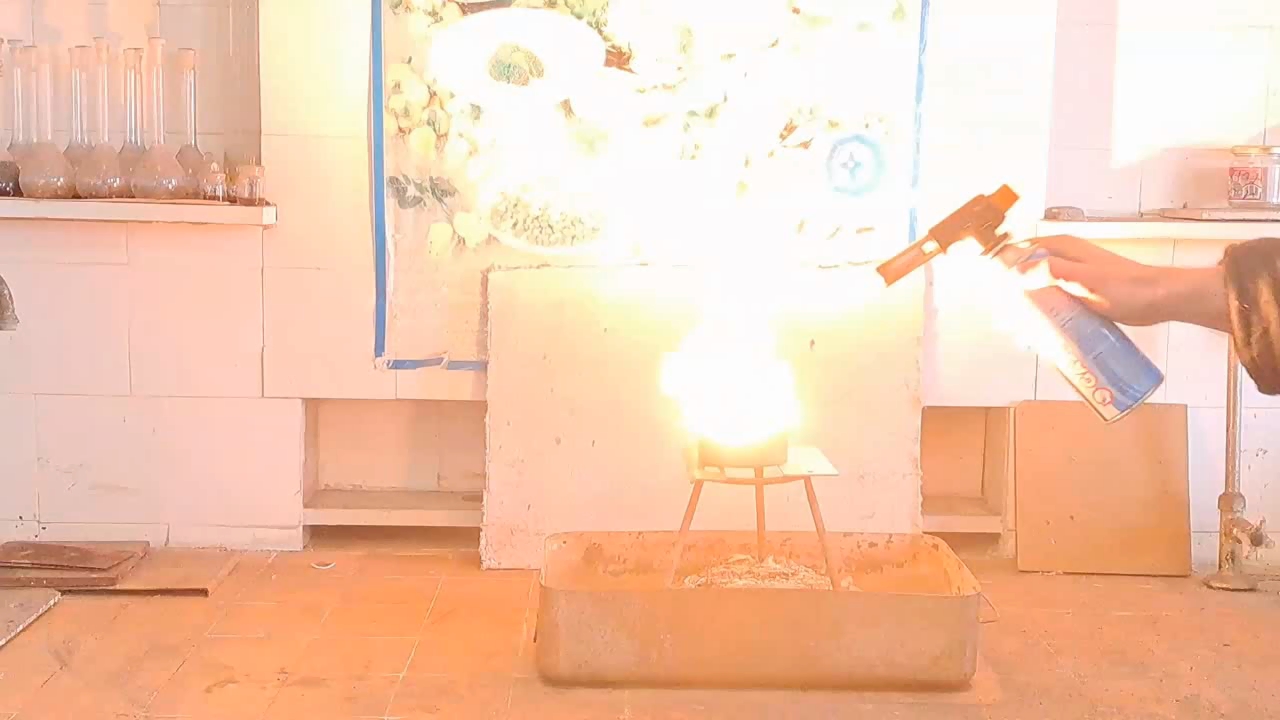
I prepared a mixture of 10 g of finely powdered aluminum and 27.5 g of iron(II, III) oxide (Fe3O4). I made a hole in the bottom of the tin can and covered it with aluminum foil to prevent the mixture from spilling out. Then I filled the can with the thermite mixture (without compacting it). I placed the can on an iron tripod, which stood in an iron tray filled with sand. I directed a strong flame from the burner at the thermite. The mixture ignited quickly, and the substances began to react vigorously. A bright yellow flame with many sparks appeared, and the tin can glowed yellow from the heat. The reaction was more intense, more violent, and lasted longer than in my previous experiments with smaller quantities. My colleagues have often told me that to properly demonstrate the thermite reaction, a sufficient amount of the mixture is needed; otherwise, the impressive visual effect is lost. Toward the end of the reaction, a stream of molten iron flowed from the bottom of the can, although it did not last long. The burning stopped, and the can cooled rapidly. I took the camera off the tripod to shoot a close-up video. At the bottom of the can, I observed red-hot droplets of porous iron; most of the metal had remained inside. The bottom of the can had burned through in several places, so the molten iron was leaking not only through the original hole, but also through new ones that had formed. |

Combustion of Thermite: Iron(II, III) Oxide/Aluminum (Fe3O4/Al) |

|

|

|

|

|

|

|

|

|

|

|

|
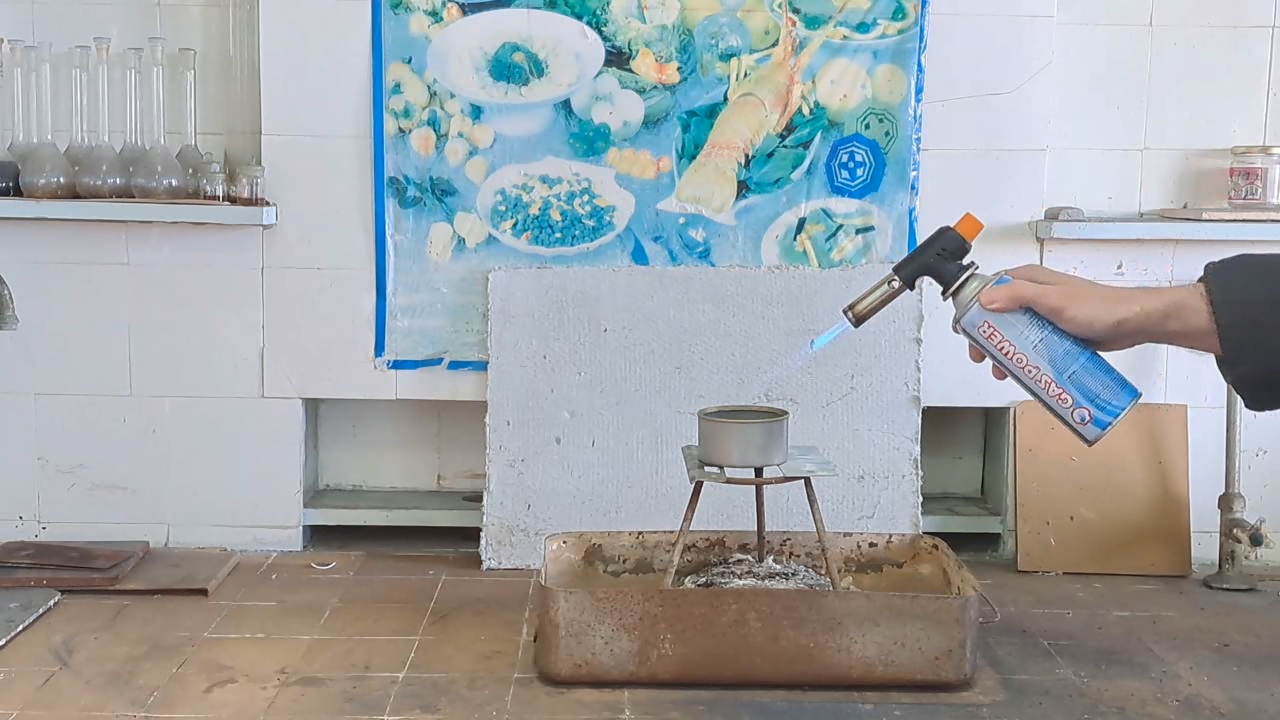
|
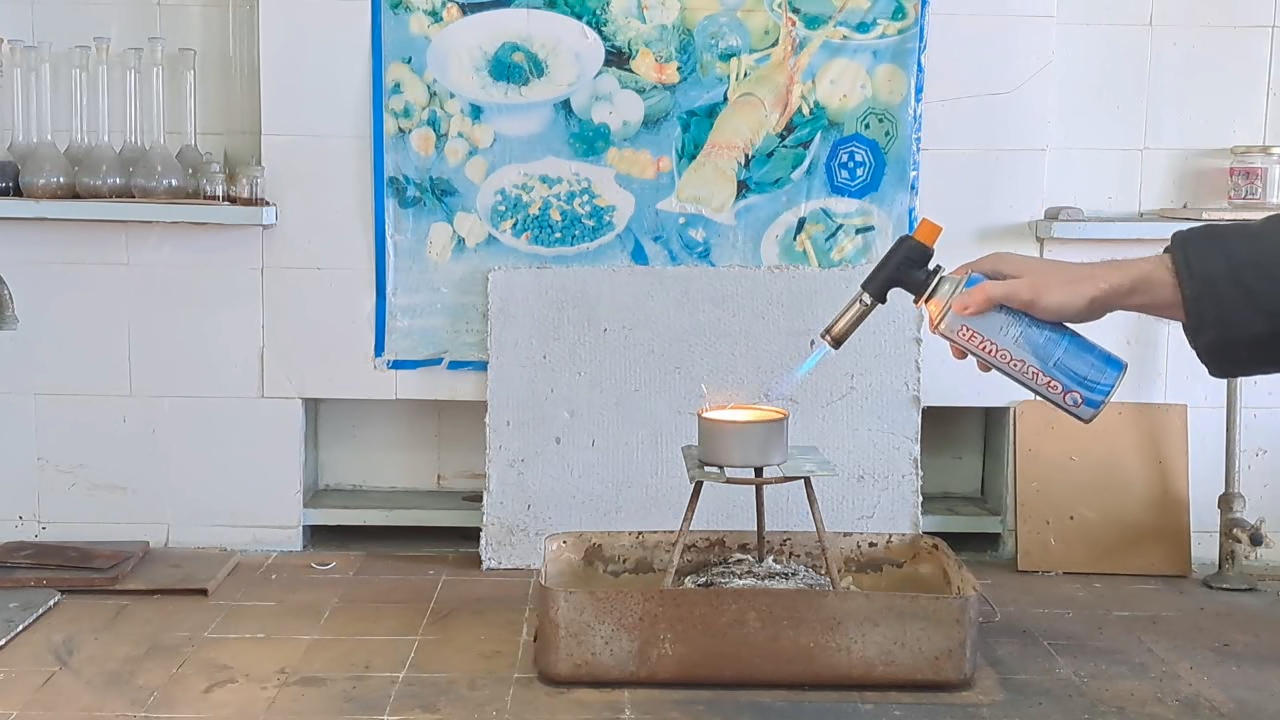
|
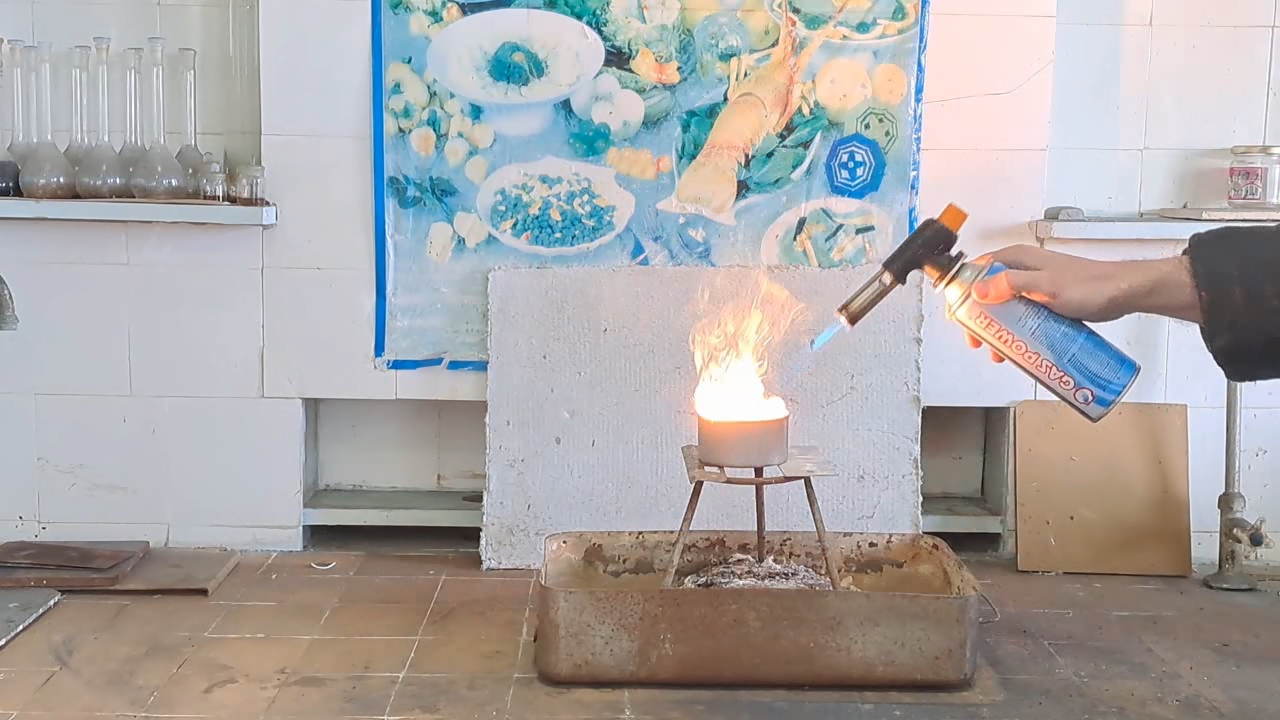
|
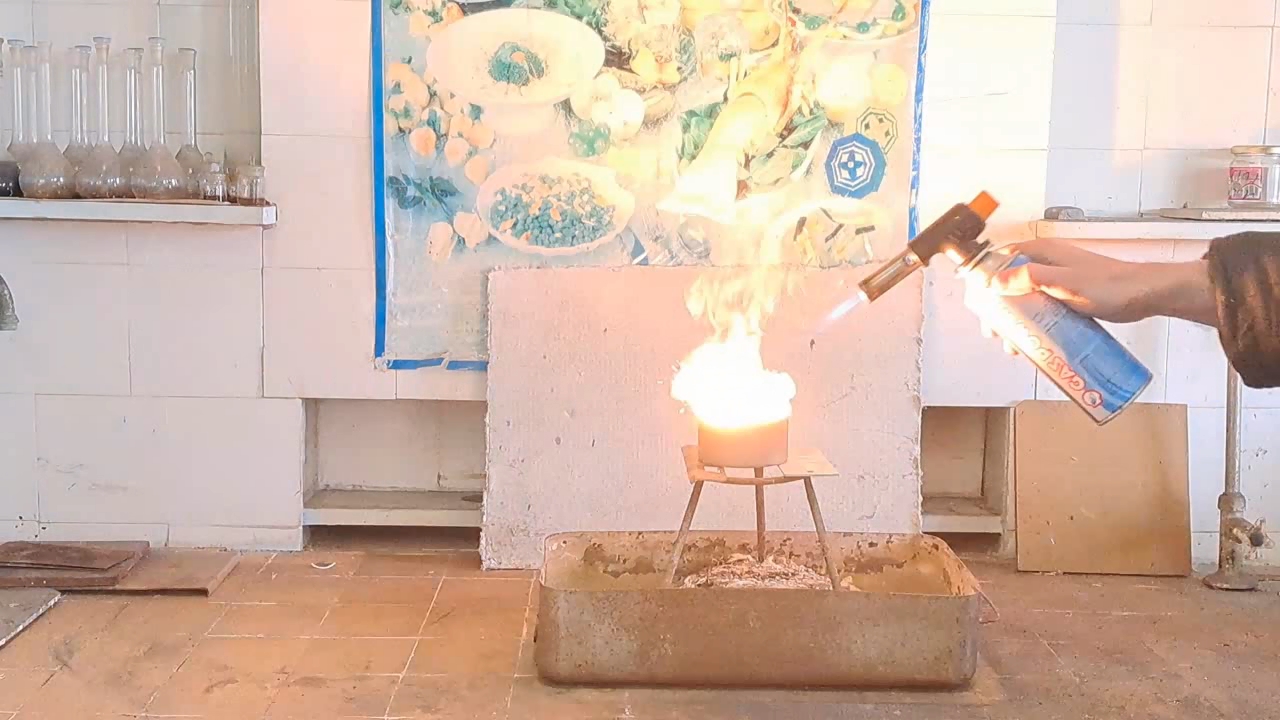
|
|
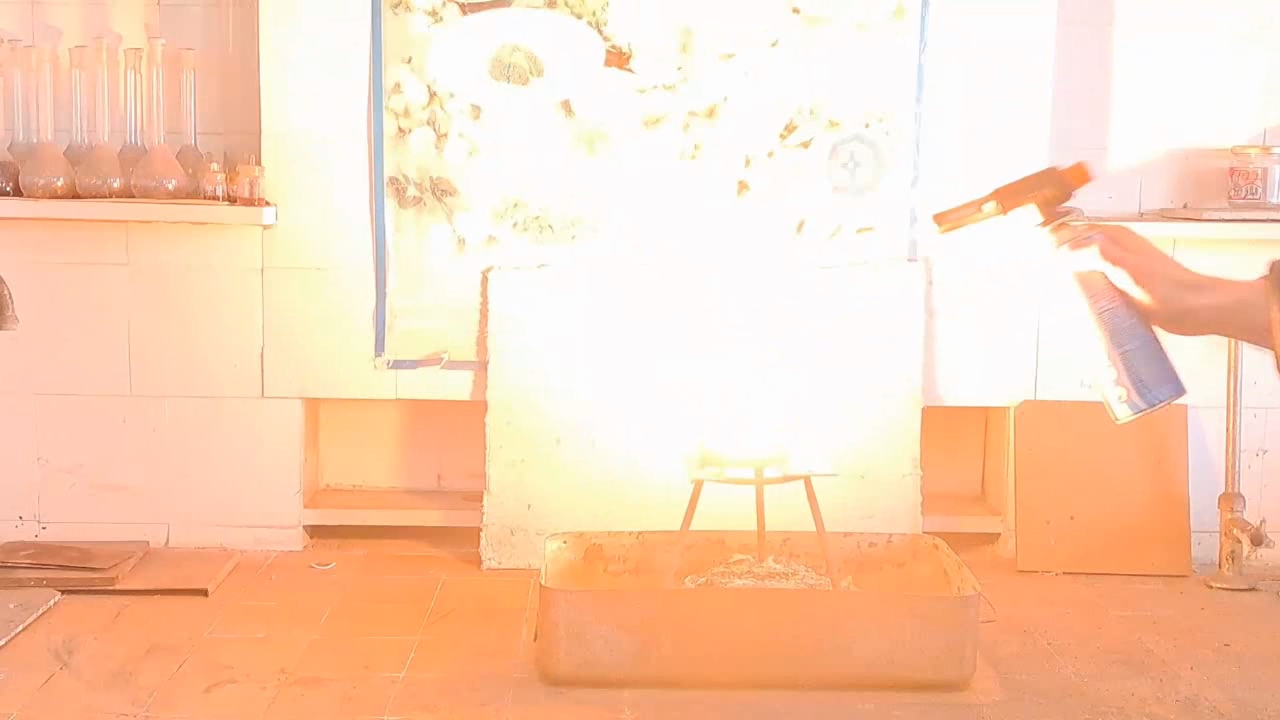
|

|

|
|
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
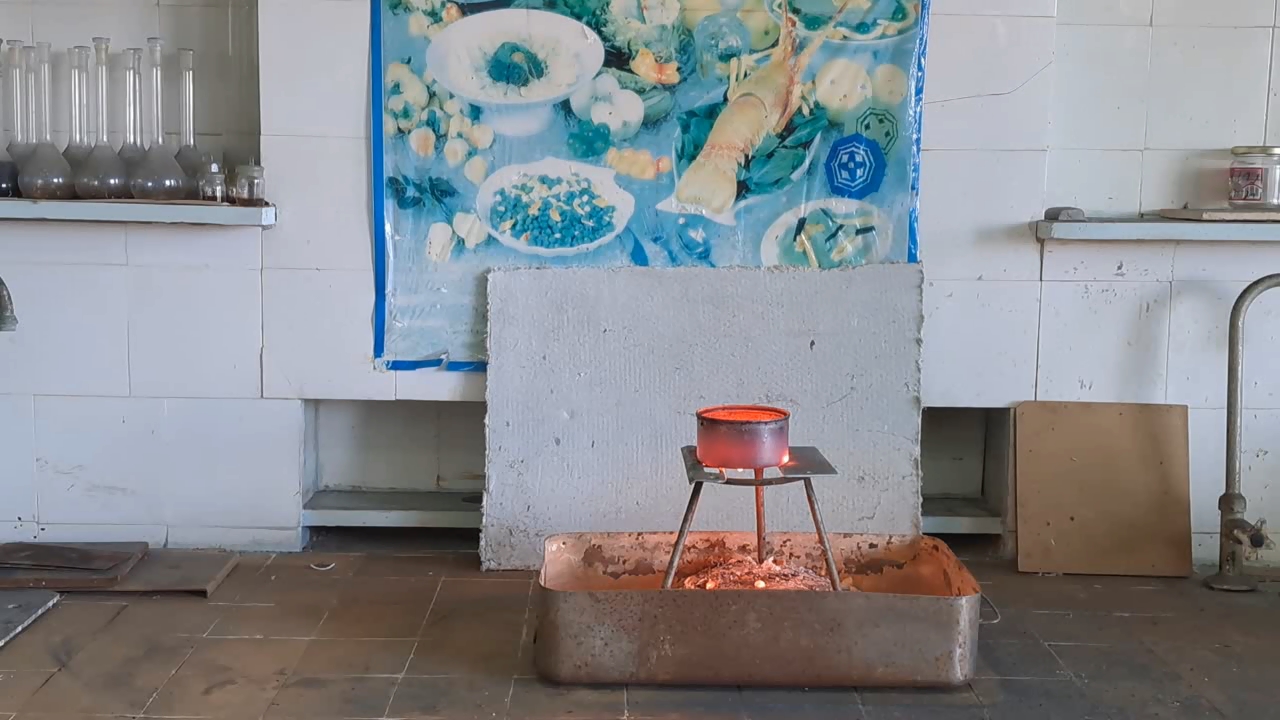
|

|

|

|

|

|

|

|

|

|

|

|

|
|
Having noticed a mistake in the text, allocate it and press Ctrl-Enter
Combustion of Thermite: Iron(II, III) Oxide/Aluminum (Fe3O4/Al) - Part 10
I increased the amount of thermite components to 15 g of finely dispersed aluminum and 41.25 g of iron(II, III) oxide (Fe3O4). The thermite was not compacted.
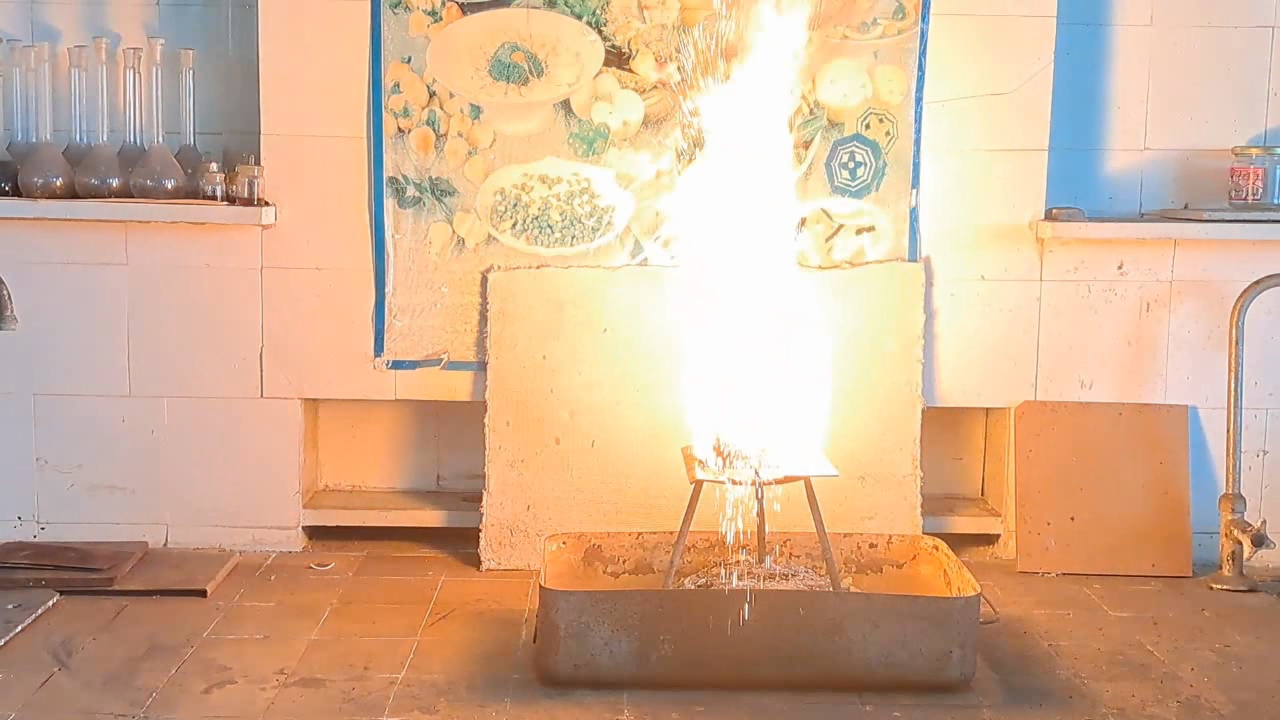
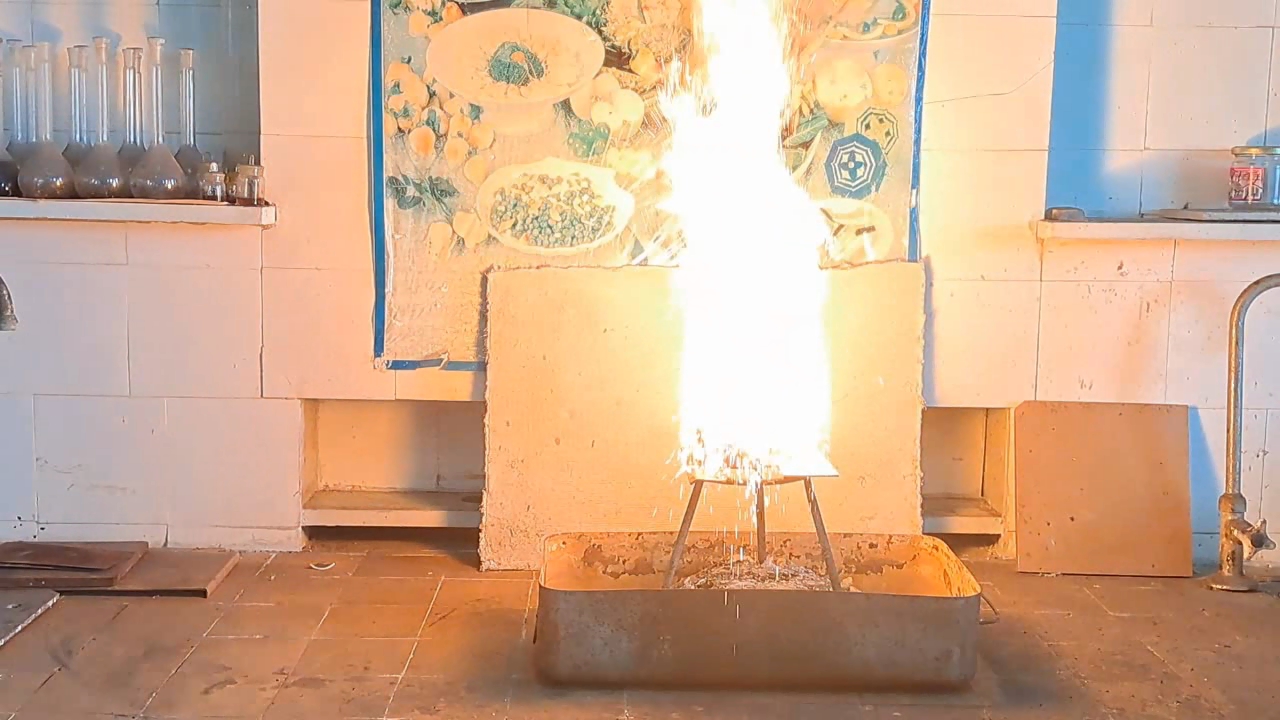
Горение термита: оксид железа (II, III)/алюминий (Al/Fe3O4) - часть 10 The rest of the experimental conditions were similar to the previous ones, except that the tin can used was narrower and taller. As a result, I managed to ignite the thermite only on the third attempt. The issue was not with the thermite itself, but with the burner and the can: when I tilted the burner downward to direct the flame into the tall can, the flame extinguished due to the poor design of the gas burner. Once I succeeded in directing the flame at the thermite, it ignited instantly. I had to urgently move my face away from the flame—otherwise, even the plexiglass shield might not have protected me from burns. A fiery fountain erupted from the can: a tall yellow flame accompanied by a shower of sparks. The walls of the tin can heated up quickly. I had hoped that a stream of molten iron would flow from the hole in the can, but instead, only numerous sparks emerged, along with occasional drops of iron. There was no continuous stream of molten metal. The walls of the can glowed yellow, but almost no iron flowed out. Gradually, the flame subsided—the thermite reaction was coming to an end. Despite the combustion having stopped, the bottom of the can remained glowing yellow for several seconds before beginning to cool rapidly. I picked up my phone and approached the can, continuing to record. Inside, I found two large "drops" of iron that had already solidified but were still glowing brightly. The thermite had burned several additional holes in the bottom of the can, but the molten metal did not pour out through them; instead, it solidified inside. In future experiments, it will be necessary to provide thermal insulation for the walls and bottom of the can to reduce heat loss. I hope that insulation will help keep the iron molten longer and allow it to flow through the hole in the bottom. A ceramic pot would have been ideal, but I didn't have one of the right size. However, I did have tin cans and clay. |

Combustion of Thermite: Iron(II, III) Oxide/Aluminum (Fe3O4/Al) |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|