Chemistry and Chemists № 2 2025
Journal of Chemists-Enthusiasts
| Content | Chemistry experiments - video | Physics experiments - video | Home Page - Chemistry and Chemists |
|
Chemistry and Chemists № 2 2025 Journal of Chemists-Enthusiasts |
Experiments with Thermite - pt.11, 12 Chemist |
|
Having noticed a mistake in the text, allocate it and press Ctrl-Enter
Combustion of Thermite: Iron(II, III) Oxide/Aluminum (Fe3O4/Al) - Part 11
I found the clay needed to insulate the tin can near my house. The soil in our area is clayey, which is inconvenient because, after rain, the clay retains water for a long time and turns into sticky mud. However, for the thermite experiments, the locally available clay was just right.
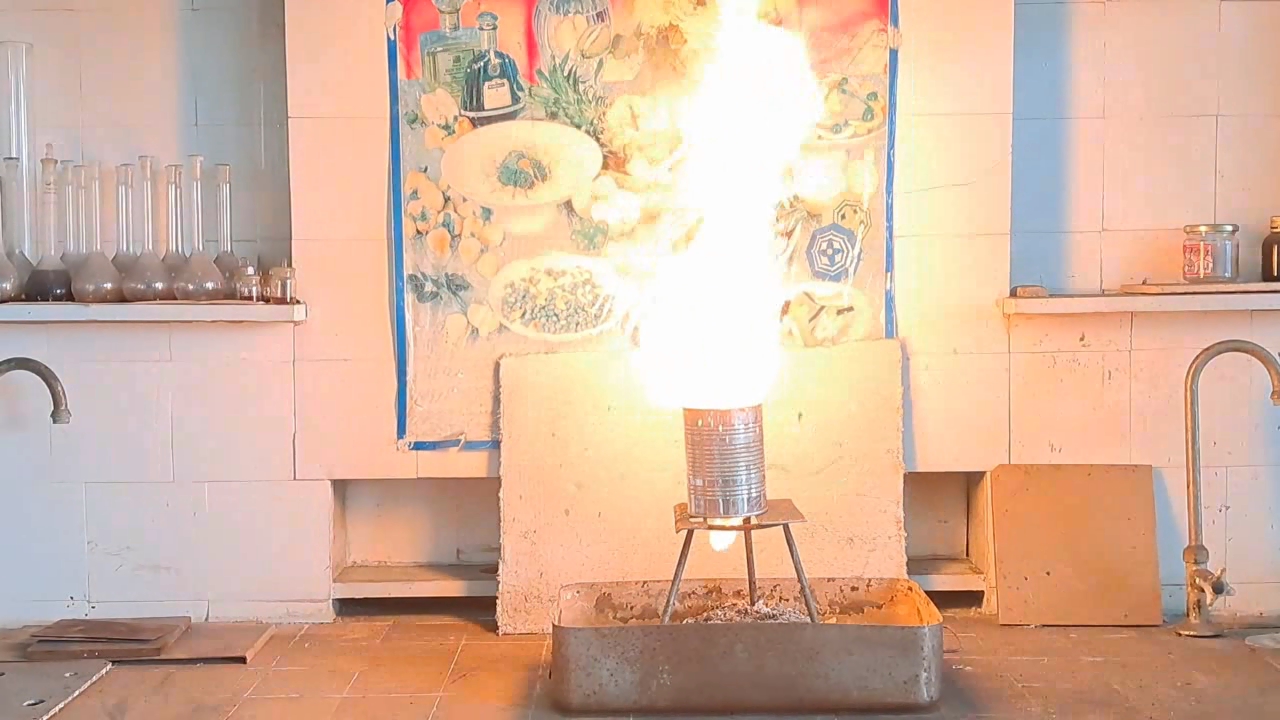
Горение термита: оксид железа (II, III)/алюминий (Al/Fe3O4) - часть 11 I mixed the clay with water, forming a dough-like mass. I coated the inner walls and bottom of the can with a thick layer of this mass, then made a hole in the bottom for the molten iron to escape. I covered the hole with aluminum foil. The can was then placed in a drying cabinet at 200°C to remove water from the clay. After that, I intended to calcine the can at a higher temperature (around 500-600°C) to remove the remaining water more thoroughly. However, I didn't have access to a muffle furnace. Later, I found out that there was no working muffle furnace in the entire institute. Therefore, I had to settle for heating the can at 200°C, hoping that during thermite combustion, the clay would not release too much water vapor, as this could splash the burning mixture or even cause an explosion. As in the previous experiment, I prepared a mixture of 15 g of finely dispersed aluminum and 41.25 g of iron(II,III) oxide (Fe3O4). It turned out that there was little free space left inside the can, as I had applied too thick a layer of clay. So, when pouring in the thermite, I had to compact the mixture. I placed the can on an iron tripod and partially covered its top with a tin lid. I directed a burner flame at the thermite. The mixture ignited immediately. A yellow flame with numerous sparks shot out of the top of the can. Large drops of molten iron flowed from the bottom hole. However, the combustion soon stopped. The last drop of iron solidified before it could detach from the bottom of the can. Upon inspection, it was clear that most of the iron formed during the thermite reaction remained inside the can. Therefore, to increase the amount of molten iron flowing out, the mass of the thermite mixture must be increased to release more heat during the reaction. |

Combustion of Thermite: Iron(II, III) Oxide/Aluminum (Fe3O4/Al) |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
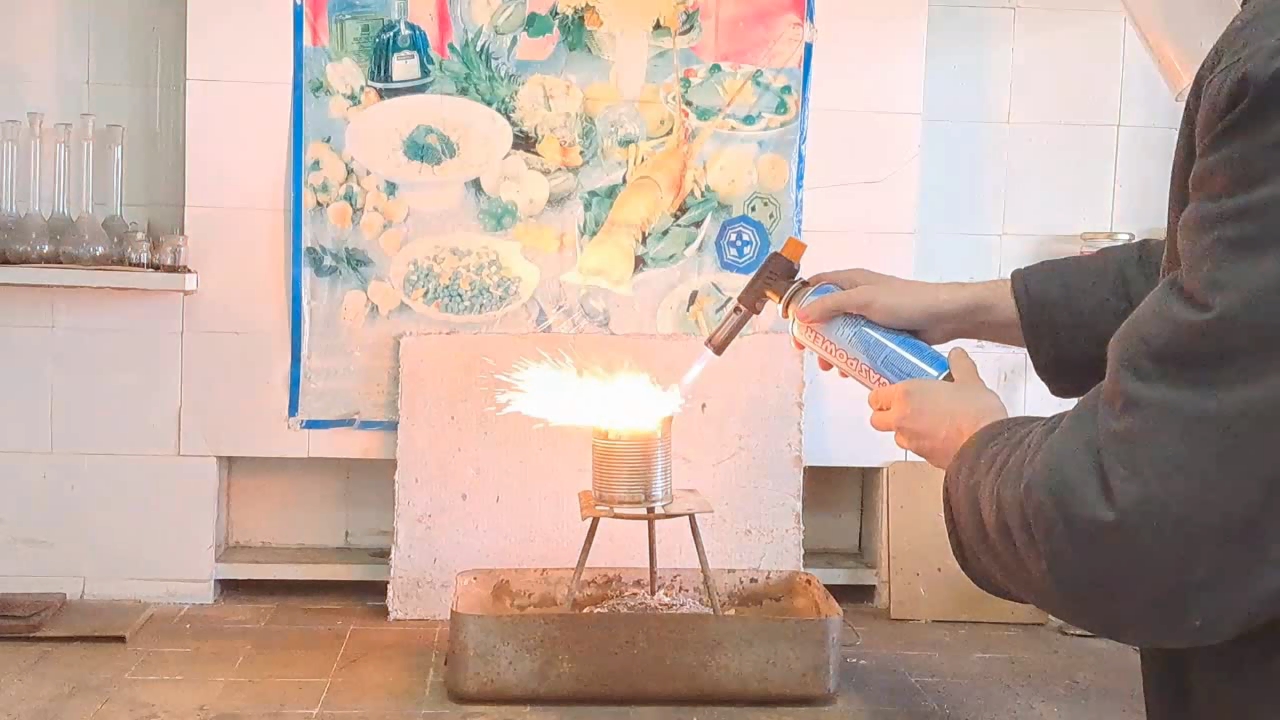
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
Having noticed a mistake in the text, allocate it and press Ctrl-Enter
Combustion of Thermite: Iron(II, III) Oxide/Aluminum (Fe3O4/Al) - Part 12
To prepare the thermite, I weighed 30 g of finely dispersed aluminum and 68.1 g of iron(II, III) oxide (Fe3O4), then thoroughly mixed the components. The weight ratio of the components, as in previous experiments, should have been 1 Al : 2.75 Fe3O4.
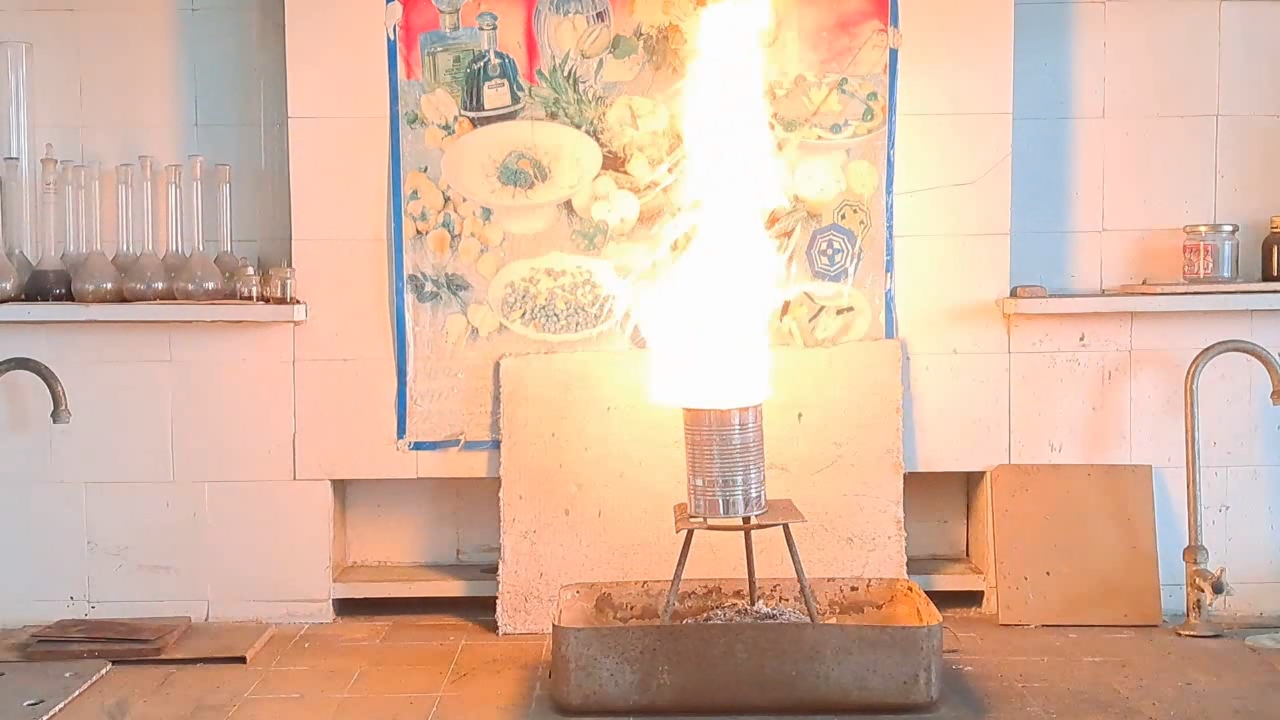
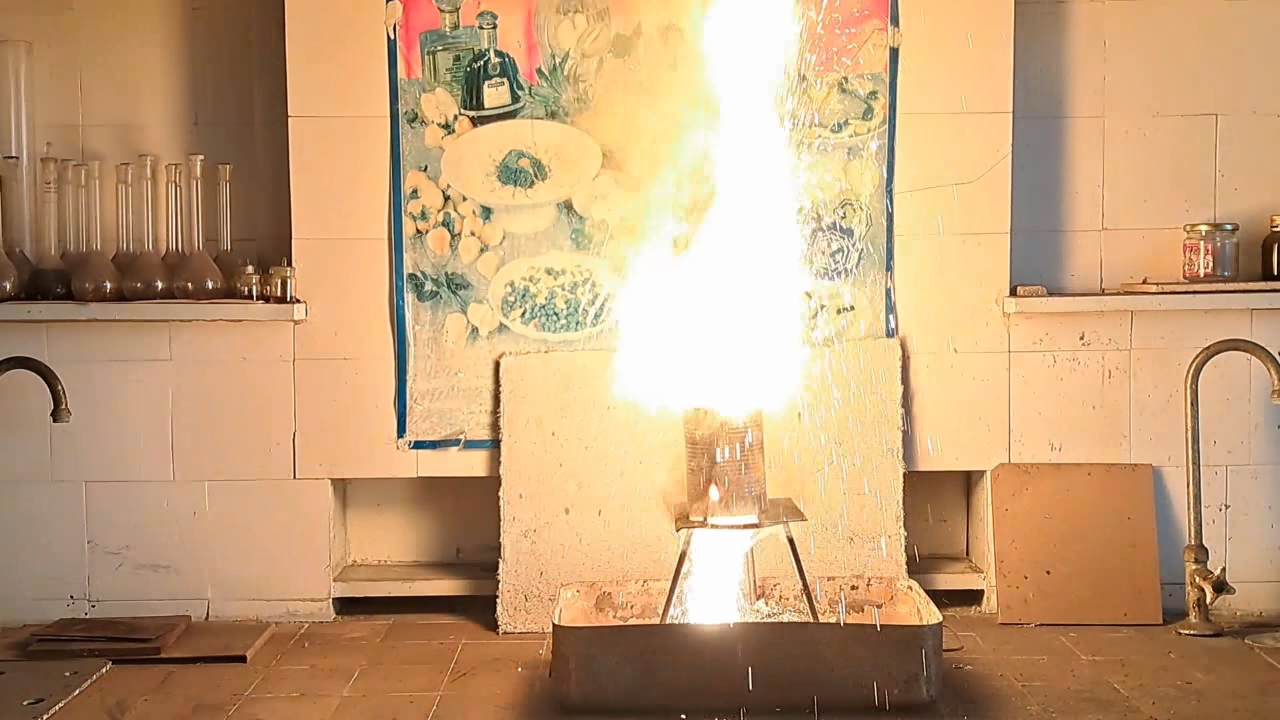
Горение термита: оксид железа (II, III)/алюминий (Al/Fe3O4) - часть 12 Only a month after the experiment did I discover a gross calculation error. I had weighed 30 g of aluminum; therefore, I should have multiplied this mass by the coefficient 2.75 to calculate the required amount of iron(II, III) oxide: 30 x 2.75 = 82.5 g. Thus, it was necessary to use 82.5 g, not 68.1 g, of Fe3O4. As a result, there was an excess of aluminum. I found the cause of the error by analyzing my lab journal. Next to the calculations was the number of the room, "223", to which I had to bring the test tubes for the centrifuge. I confused this number with the coefficient "2.23" and multiplied the mass of aluminum by it, obtaining an erroneous value for the mass of iron oxide (68.9 g). I covered the inside of a large tin can with clay. The clay layer was thin. To be honest, I used the leftover clay dough from the previous experiment. As in the previous case, I dried the can with clay in a thermostat at 200°C and made a hole in the bottom of the can. I wrapped the thermite in paper without compacting it and then placed the package in the can. I placed the can with thermite on an iron tripod, partially covered it with a tin lid, and directed the flame of the burner at the thermite. A tall yellow flame shot out of the top of the can, and sparks flew. There was a hissing sound. A little later, a flame also shot out of the hole in the bottom of the can. Vigorous combustion continued for about 15 seconds. In this experiment, the flame was the tallest. During other experiments with thermite (both earlier and later), the flame was much lower. In particular, even when I used more thermite, the flame height was still lower. I was seriously concerned that the top of the fume hood might catch fire. Sparks poured out of the bottom of the can, but there were no streams or large drops of molten iron. The tin can was glowing yellow in one spot; apparently, the insulation had burned through there. When I came closer, I saw yellow-hot metal at the bottom and sides of the can, but it never leaked out. The fume hood was covered with dark particles ejected by the burning thermite. In addition to the calculation error, a colleague pointed out another reason for the failure: I had not compacted the thermite. As is known, gases are not formed when thermite burns. However, in uncompacted thermite, there is a lot of air between the solid particles. When the thermite burns, the air heats up and expands. As a result, the gas flow carries heat and solid particles upward. Consequently, a tall flame with many sparks was observed. However, due to the large heat loss, the stream of molten iron could not flow out of the hole in the bottom of the can. |

Combustion of Thermite: Iron(II, III) Oxide/Aluminum (Fe3O4/Al) |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
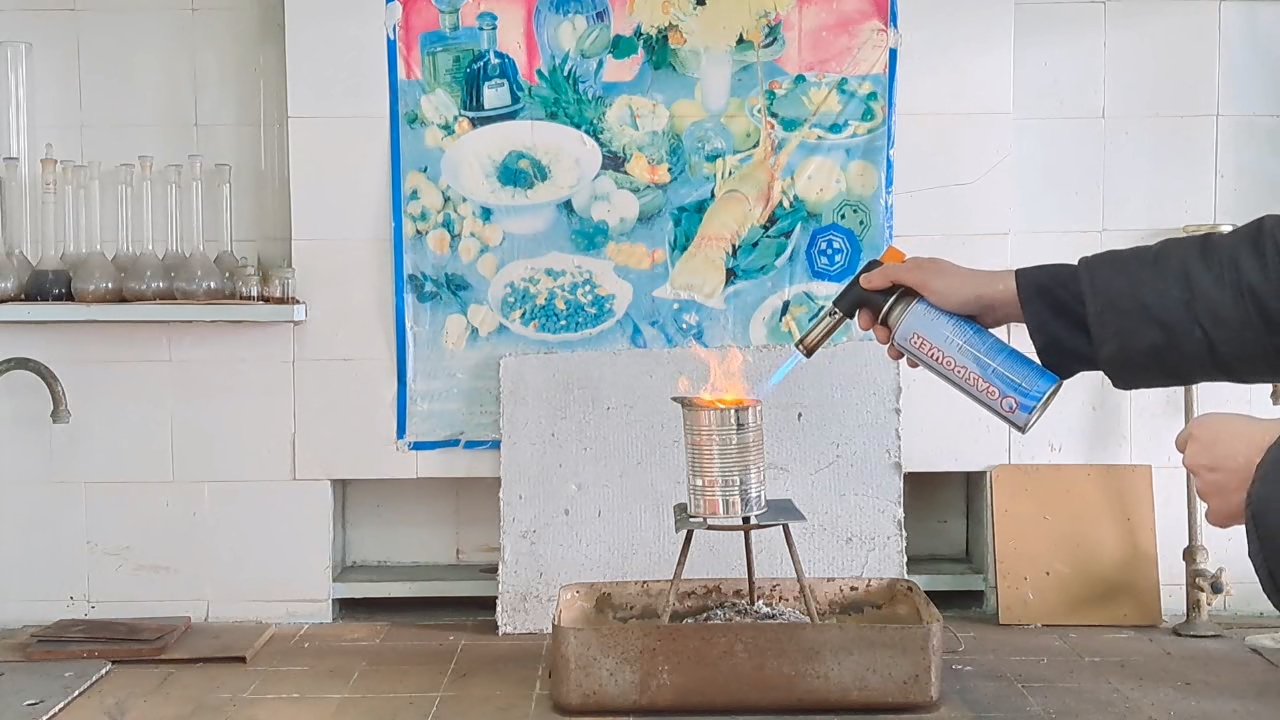
|

|
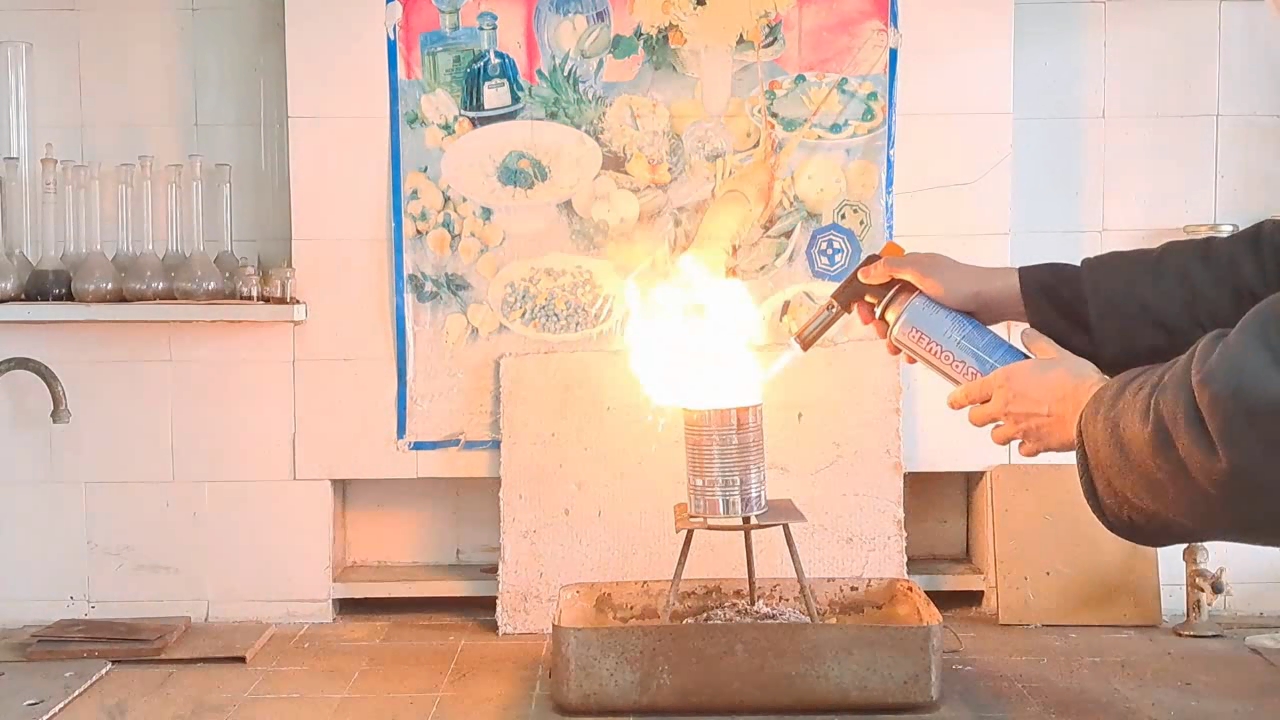
|

|

|
|
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
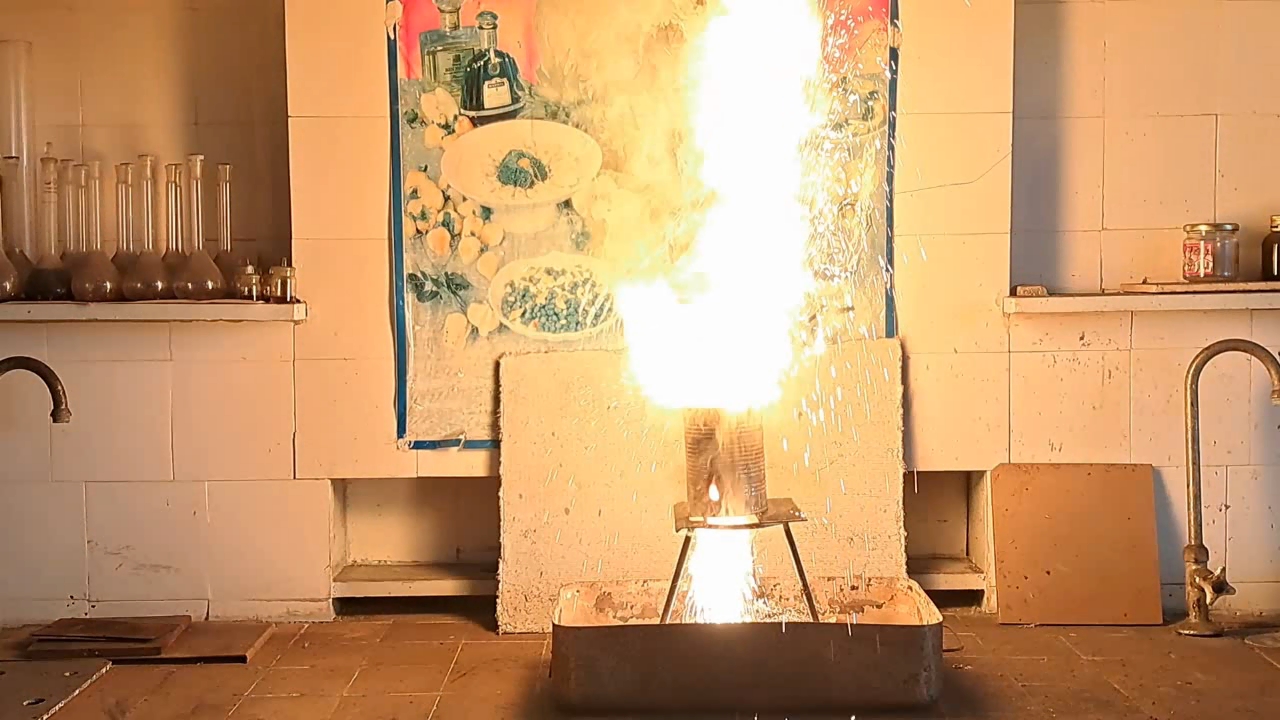
|
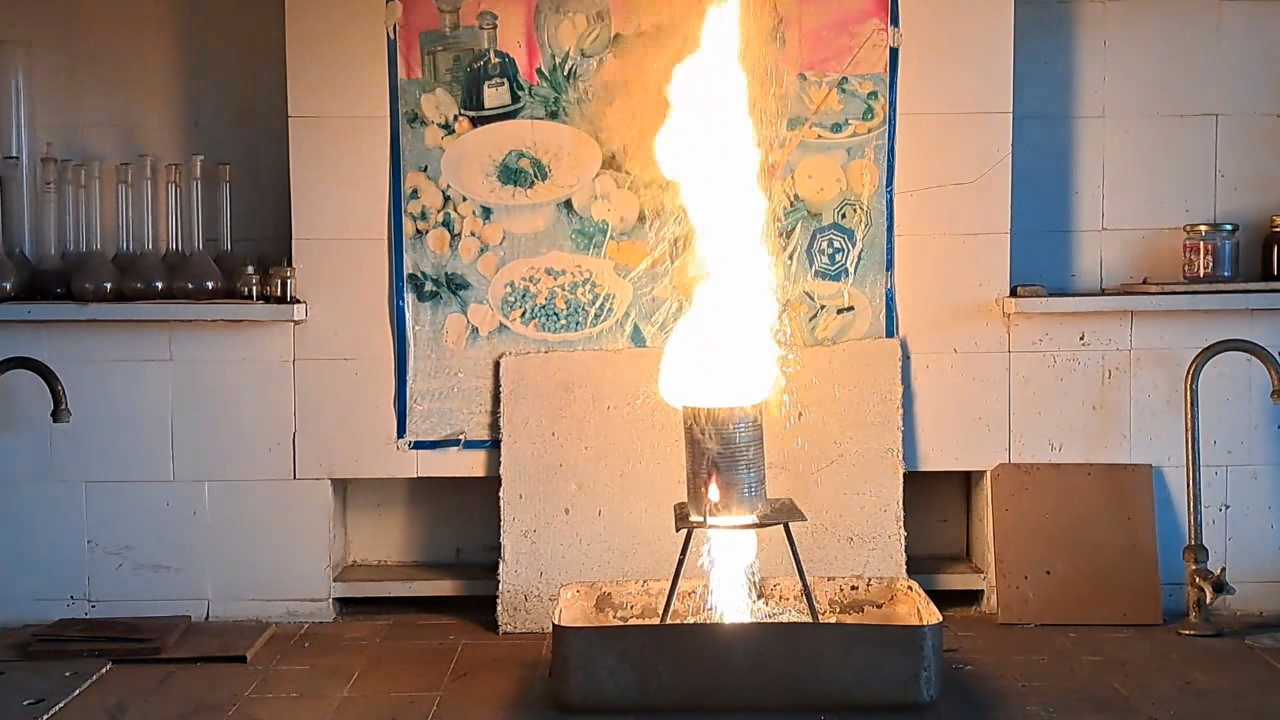
|

|

|

|

|

|

|

|

|

|

|

|
|

|

|

|