Chemistry and Chemists № 2 2025
Journal of Chemists-Enthusiasts
| Content | Chemistry experiments - video | Physics experiments - video | Home Page - Chemistry and Chemists |
|
Chemistry and Chemists № 2 2025 Journal of Chemists-Enthusiasts |
Experiments with Thermite - pt.13, 14 Chemist |
|
Having noticed a mistake in the text, allocate it and press Ctrl-Enter
Combustion of Thermite: Iron(II, III) Oxide/Aluminum (Fe3O4/Al) - Part 13
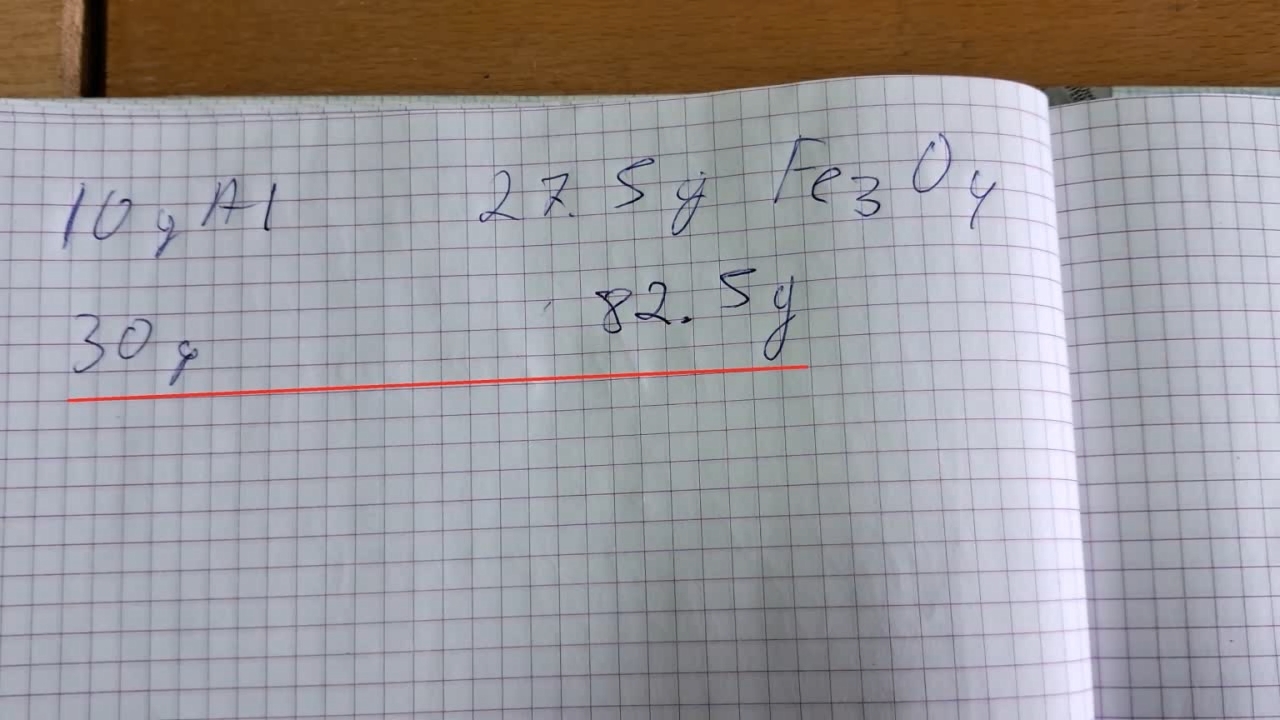
A colleague gave me a real gift—he bought two clay flower pots. The pots were the right size for conducting the thermite combustion. I prepared a mixture of 30 g of aluminum and 82.5 g of iron oxide, Fe3O4. This time, the aluminum came from a different batch. The aluminum particles were larger than in previous experiments, but the metal was still finely dispersed.
Горение термита: оксид железа (II, III)/алюминий (Al/Fe3O4) - часть 13 In fact, I prepared the same mixture I had planned to use in the last experiment, but that time, I made a mistake in the calculations. The mass of the thermite exceeded 110 g. I wanted to conduct the experiment in the laboratory (similar to the previous ones), but I was frankly afraid that uncontrolled combustion could occur, which usually ends in a large fire. Wouldn't it be better to conduct the thermite combustion outside, on a vacant lot? My concern was justified, and I am not at all embarrassed to admit that I was afraid. The fact is that the previous experiments were conducted in tin cans, and this time I had to use a clay pot. The pot could crack and fly apart into pieces, like the porcelain crucible in one of the earlier experiments. As a result, 110 g of burning mixture could be scattered around the room. I shared my concerns with the colleague. In response, he brought a plate of sand and asbestos cords. I asked: — What do you suggest I do with these items? He laid a cord between the pot and the tripod ring. It was of no use, but I did not argue. I examined the "OU-2" fire extinguisher hanging at the entrance to the lab (a CO2 fire extinguisher). There was no seal on the extinguisher, which meant it could be used without leaving traces of my actions. Excellent! I put the fire extinguisher on the table in front of me. I placed a glove next to it to avoid frostbite while holding the aluminum bell of the working carbon dioxide extinguisher. My colleague objected that thermite cannot be extinguished with a fire extinguisher, and that sand was also not suitable—it must be extinguished with dry potassium chloride. He might as well have been lecturing on the benefits of a life preserver or a lifeboat to a person floating among wreckage in the ocean. There was no potassium chloride in the lab, and certainly no "dried" potassium chloride. Most importantly, I was not planning to use the fire extinguisher to put out the thermite itself. It was intended for the objects that burning thermite might ignite. This time, I placed the thermite in a paper bag, compressed it with my hands, and placed it in the pot. I partially covered the pot with a sheet of iron. I tried to ignite the thermite with a torch flame, but the sheet interfered. I removed the iron sheet and directed a strong flame at the thermite again. There was a bright flash, and I stepped back. A bright yellow flame with many sparks shot out of the pot. The combustion resembled a sparkling pyrotechnic fountain. Almost immediately, I heard the sounds of cracking ceramics. The video clearly shows how cracks appeared in the pot's walls. Flames shot out of the cracks, and sparks rained down. Fortunately, the pot did not explode, as the crucible had earlier. It didn't even fall apart. Molten iron was flowing from a hole at the bottom, but there was not much of it. When the burning stopped, the contents of the pot were glowing brightly with a yellow light. My colleague motioned for me to pick up the smartphone and come closer, so I did. I was filming hot objects from a close distance, risking not only getting badly burned but also damaging the smartphone. The risk was worth it: a bright and very beautiful yellow light was coming through the cracks in the pot's walls. Looking from above, the inside of the pot was glowing brightly. I unexpectedly discovered that the phone camera... was turned off. It turned out I had accidentally pressed the stop recording button when I took the camera off the tripod. I restarted video recording, but by that time the mixture had partially cooled—the yellow glow had changed to red, and it was not as beautiful. I was very upset because I had lost some interesting footage. My friend encouraged me: — But you have another pot. — You're right: we need to repeat the experiment. I resisted the temptation to start a new experiment immediately; it was evening, and I was very tired. I went home feeling both excited and upset. |
Combustion of Thermite: Iron(II, III) Oxide/Aluminum (Fe3O4/Al) |

|

|

|
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
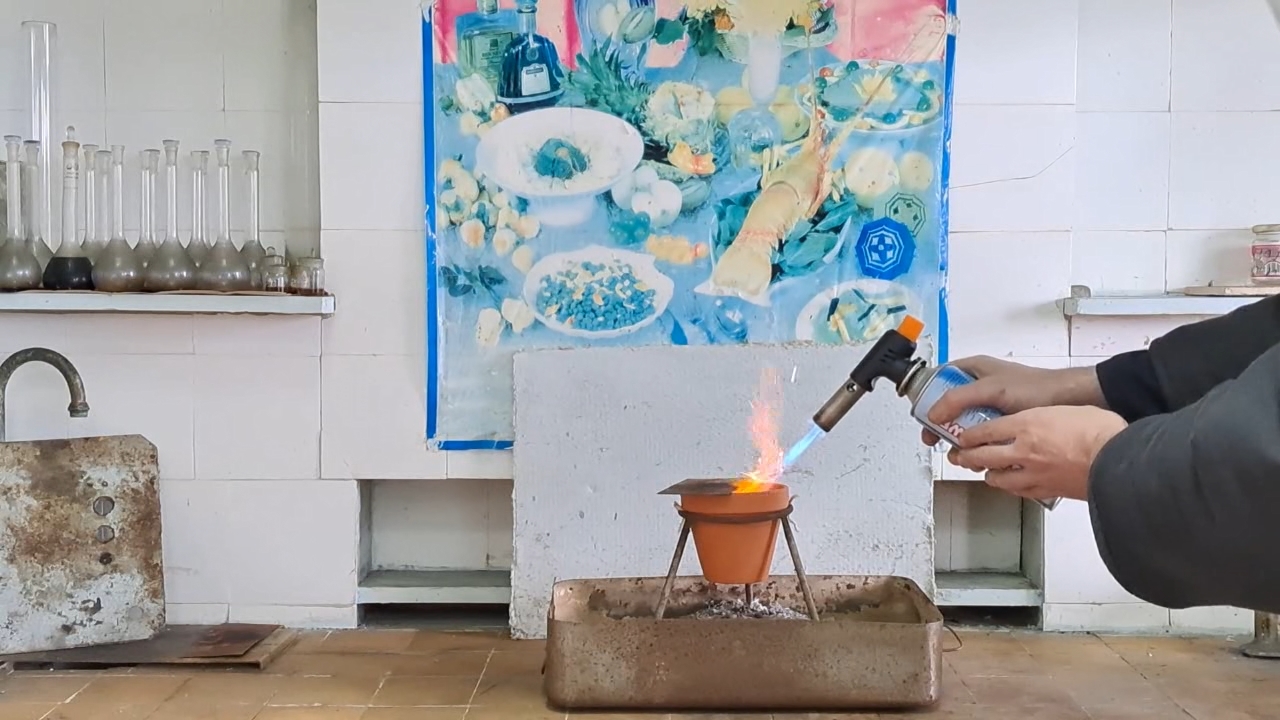
|

|

|

|

|

|
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
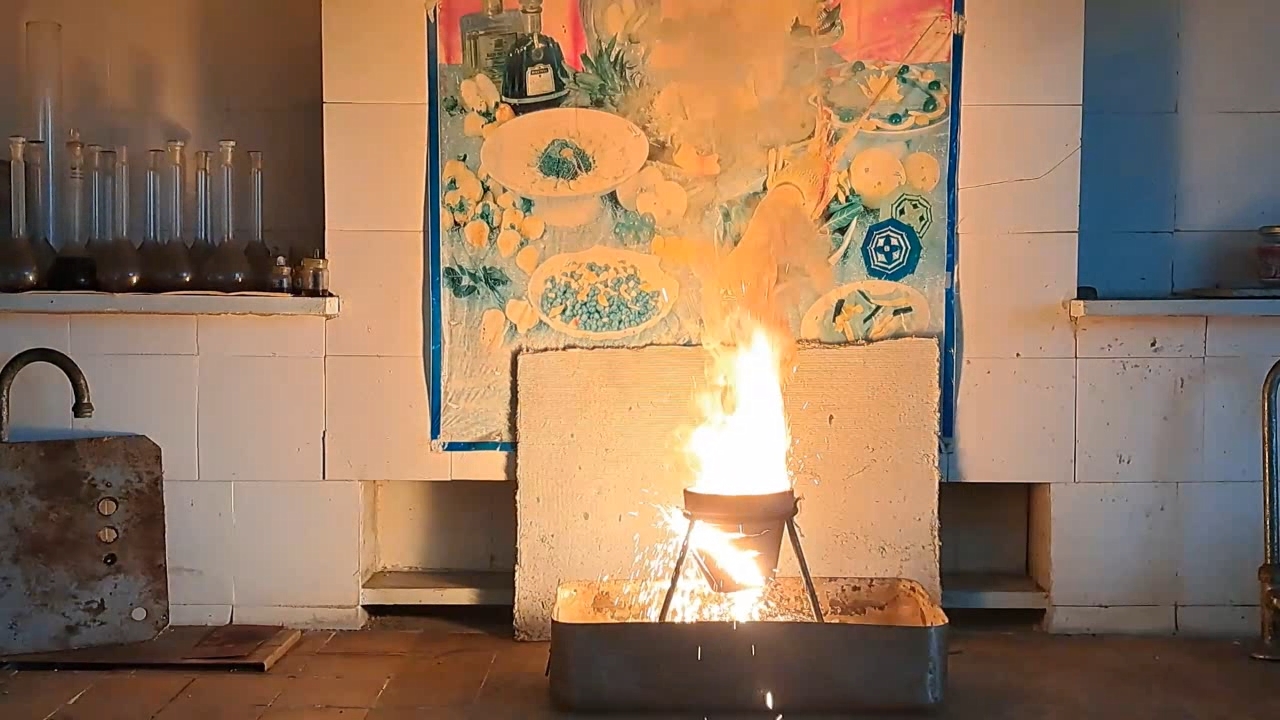
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
Having noticed a mistake in the text, allocate it and press Ctrl-Enter
Combustion of Thermite: Iron(II, III) Oxide/Aluminum (Fe3O4/Al) - Part 14
An unfortunate accident caused part of the footage from the previous experiment to be lost. Therefore, I decided to repeat the combustion of thermite in a clay pot, this time increasing the amounts of the substances. I prepared a mixture of 40 g of aluminum and 110 g of iron(II,III) oxide (Fe3O4).
Горение термита: оксид железа (II, III)/алюминий (Al/Fe3O4) - часть 14 I wrapped the thermite in a sheet of paper and compressed it tightly in my palm. Then I placed the paper-wrapped mixture into a ceramic pot mounted on an iron tripod. Separately, I prepared an incendiary mixture consisting of 1 g of finely dispersed aluminum and 2.75 g of iron(II,III) oxide (Fe3O4), which I poured on top of the main thermite charge. Everything was ready for the experiment. I understood that it would be unlikely to accurately reproduce the previous result. For example, the pot could crack on the side opposite the camera. As a result, the sparks and bright yellow light, emerging through the cracks, might not be visible in the video. The bottom of the pot might fall off, or other unforeseen events could occur. I decided to proceed without worrying about factors beyond my control. I aimed the burner flame at the thermite—the mixture ignited. A tall yellow flame with many sparks erupted from the pot, accompanied by a hissing sound. The sound of cracking ceramic followed. As I had anticipated, the first cracks appeared outside the camera's field of view. I could see them from a different angle, but they weren't captured in the video. A few seconds later, the cracks widened and became clearly visible on camera. Flames and sparks burst from the cracks, and the bottom of the pot detached. Fortunately, almost all of the yellow-hot mass fell into a tray filled with sand. This time, I wasn't afraid but still kept a fire extinguisher close at hand. I removed the camera from the tripod, approached the setup, and filmed from a close distance. The detached bottom glowed bright yellow, filled with molten iron that slowly cooled with a crackling sound. This time, I managed to capture the entire experiment on video. However, the pot's bottom fell off. It became evident that ceramic pots of this type are poorly suited for demonstrating thermite combustion. |

Combustion of Thermite: Iron(II, III) Oxide/Aluminum (Fe3O4/Al) |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|