Chemistry and Chemists № 2 2025
Journal of Chemists-Enthusiasts
| Content | Chemistry experiments - video | Physics experiments - video | Home Page - Chemistry and Chemists |
|
Chemistry and Chemists № 2 2025 Journal of Chemists-Enthusiasts |
Experiments with Thermite - pt.15, 16 Chemist |
|
Having noticed a mistake in the text, allocate it and press Ctrl-Enter
Combustion of Thermite: Fe3O4 + KNO3 + Al (waste) - Part 15
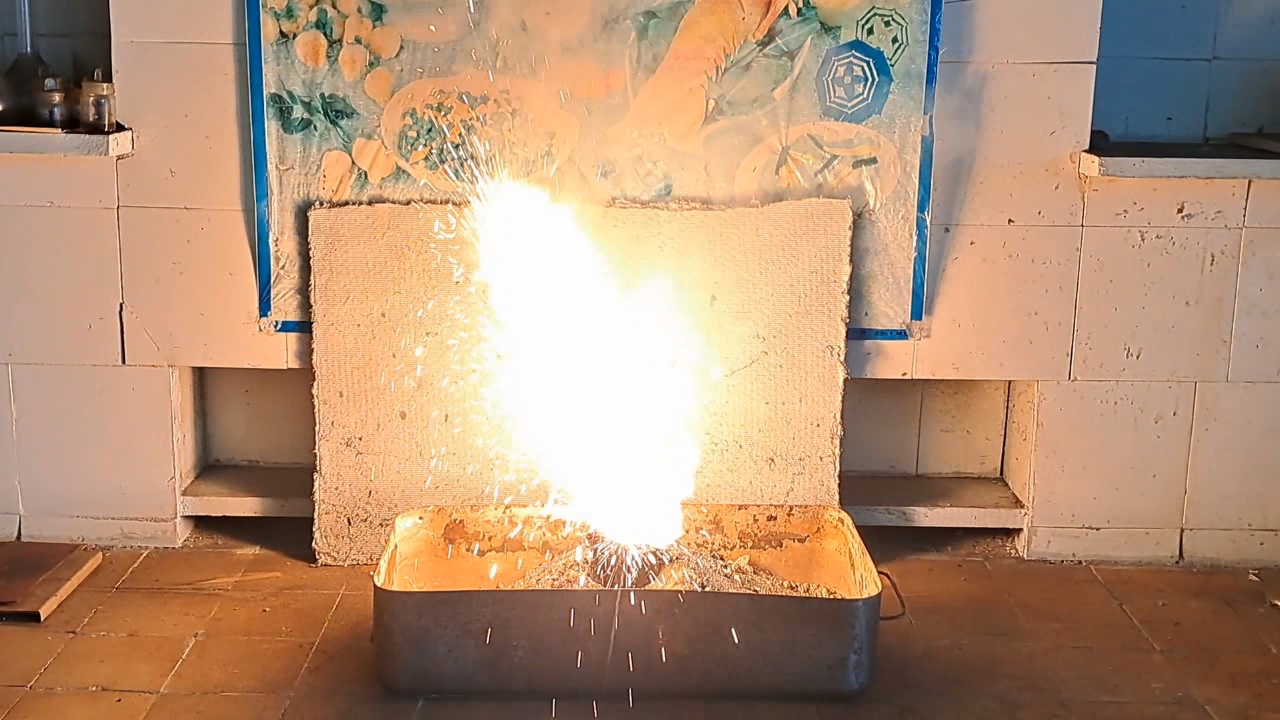
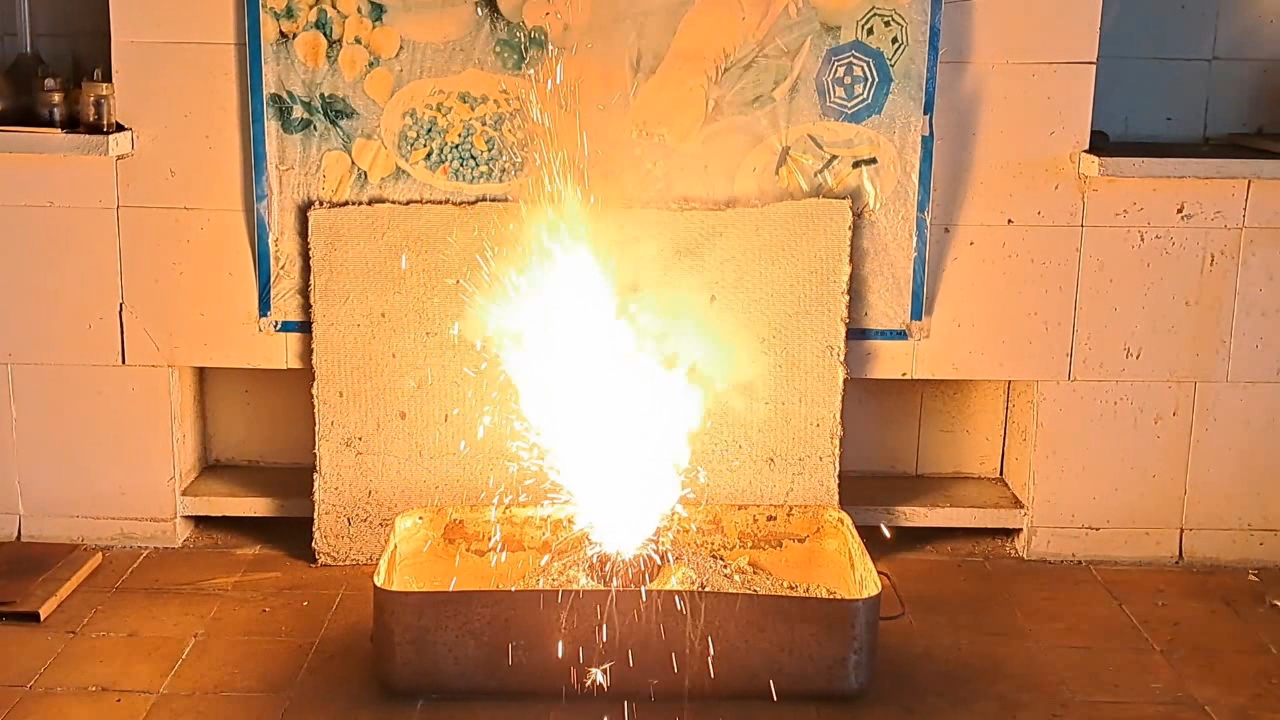
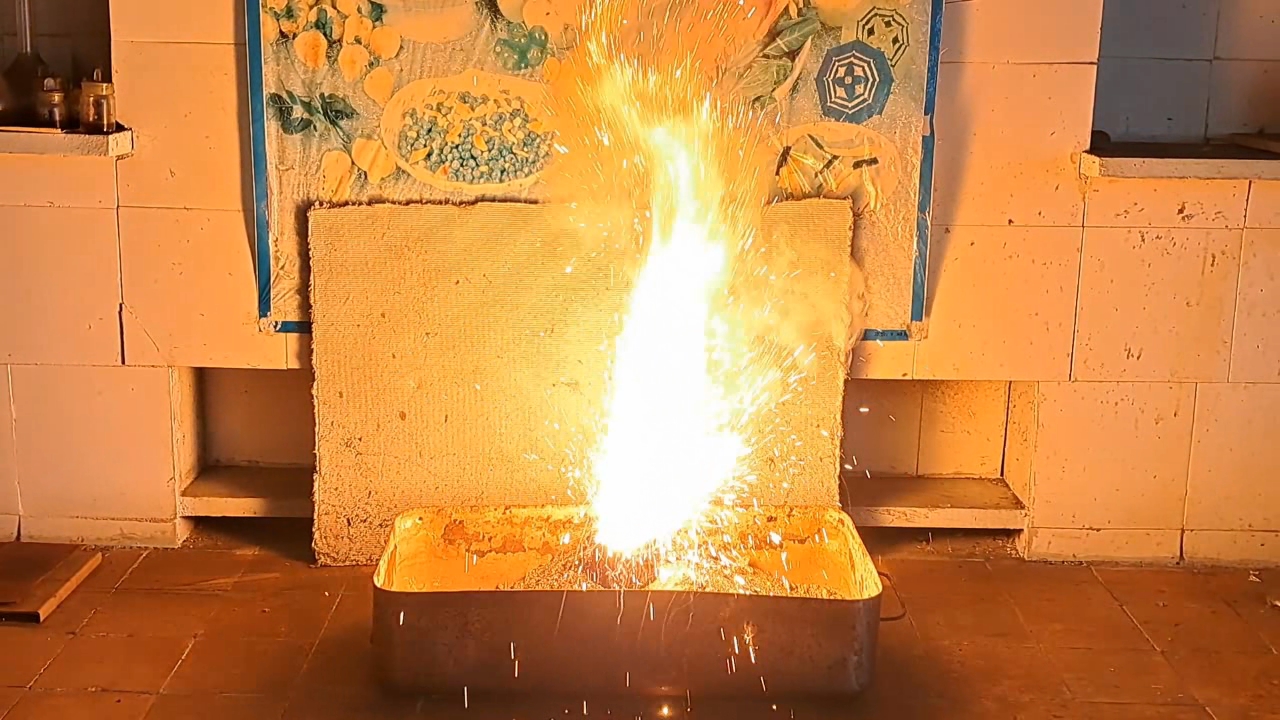
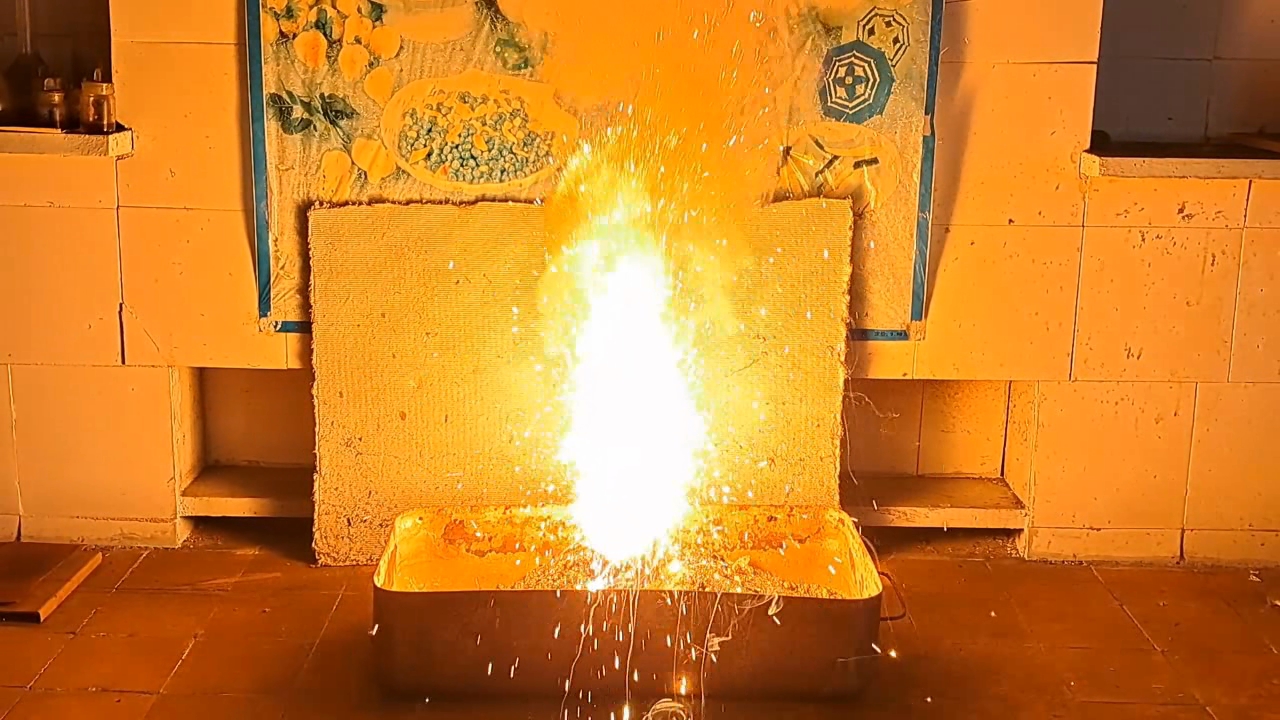
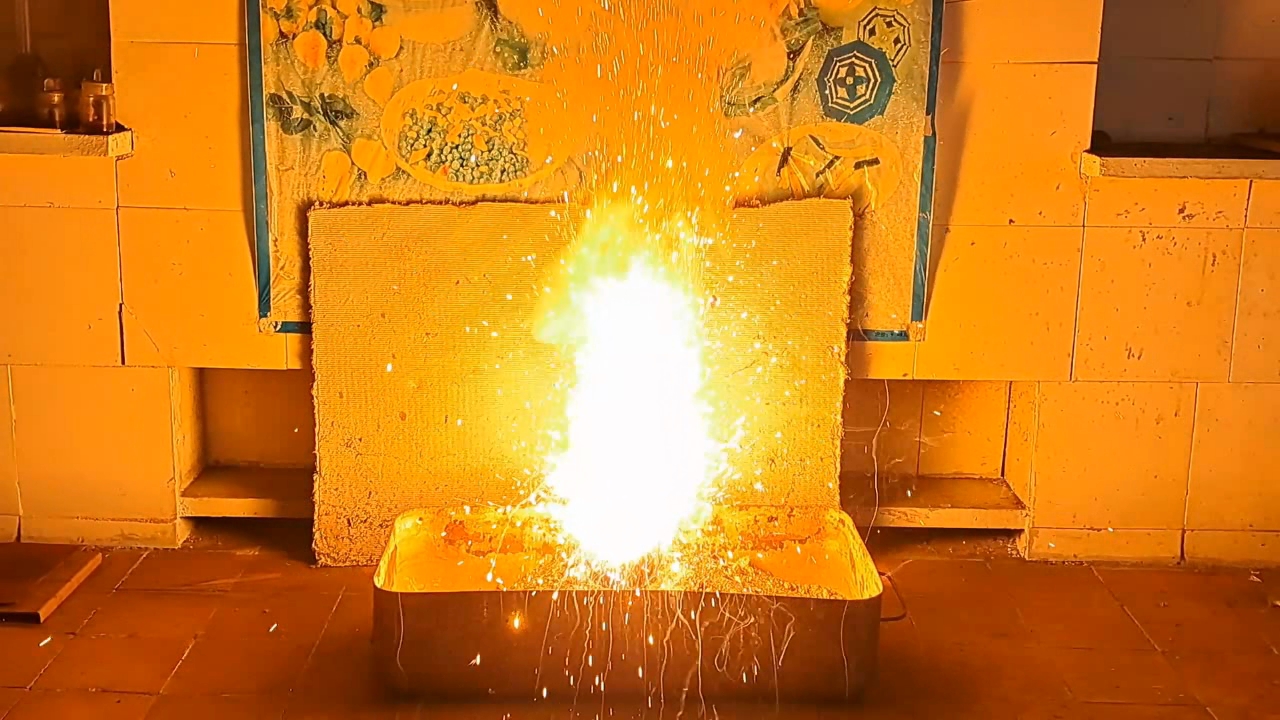
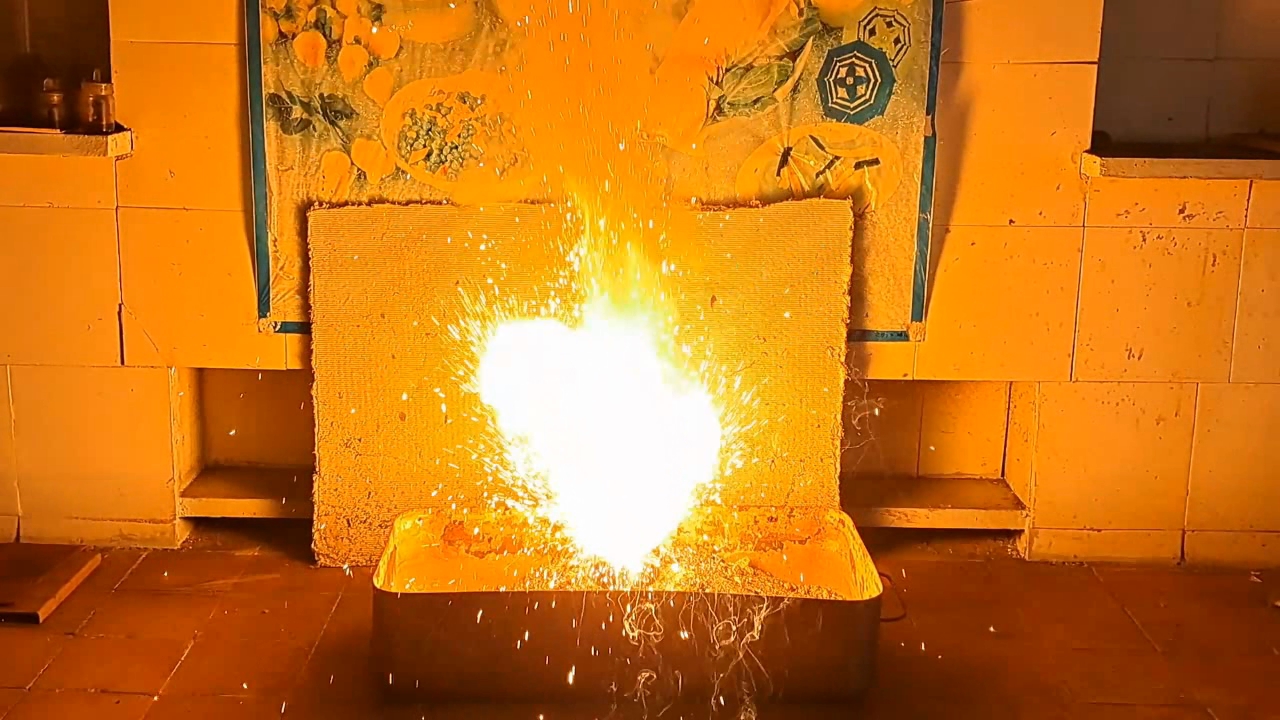
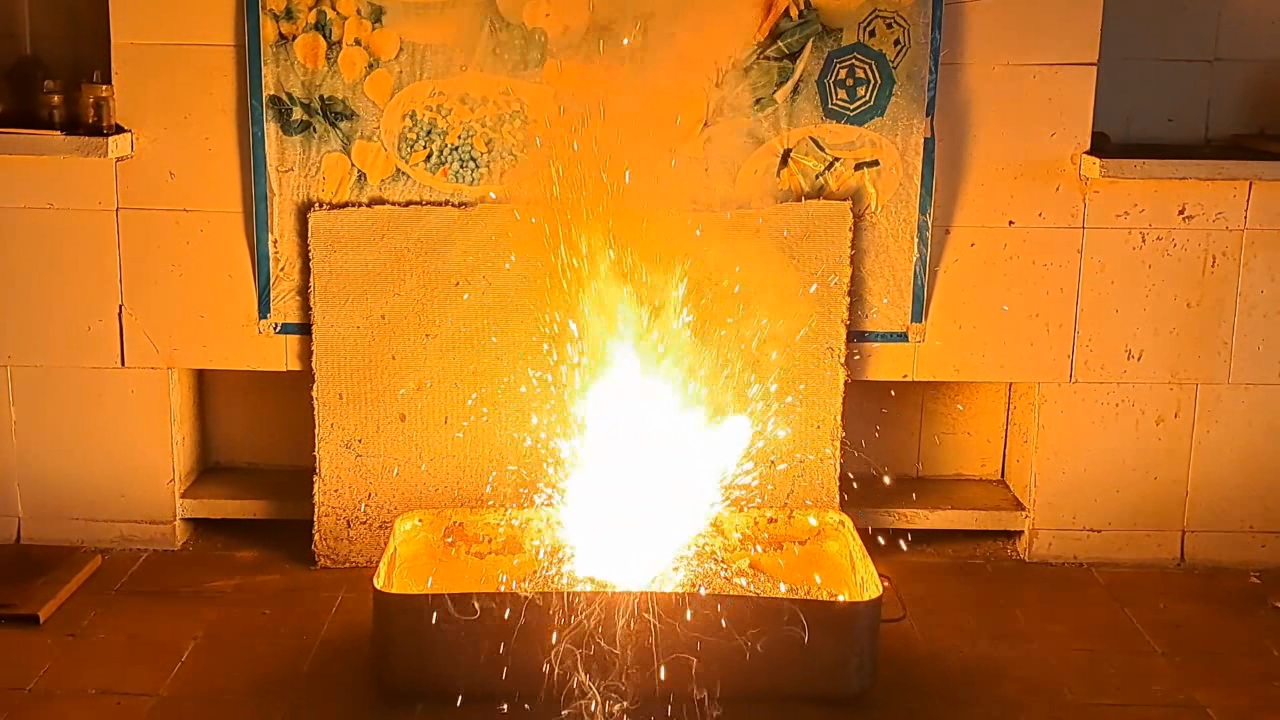
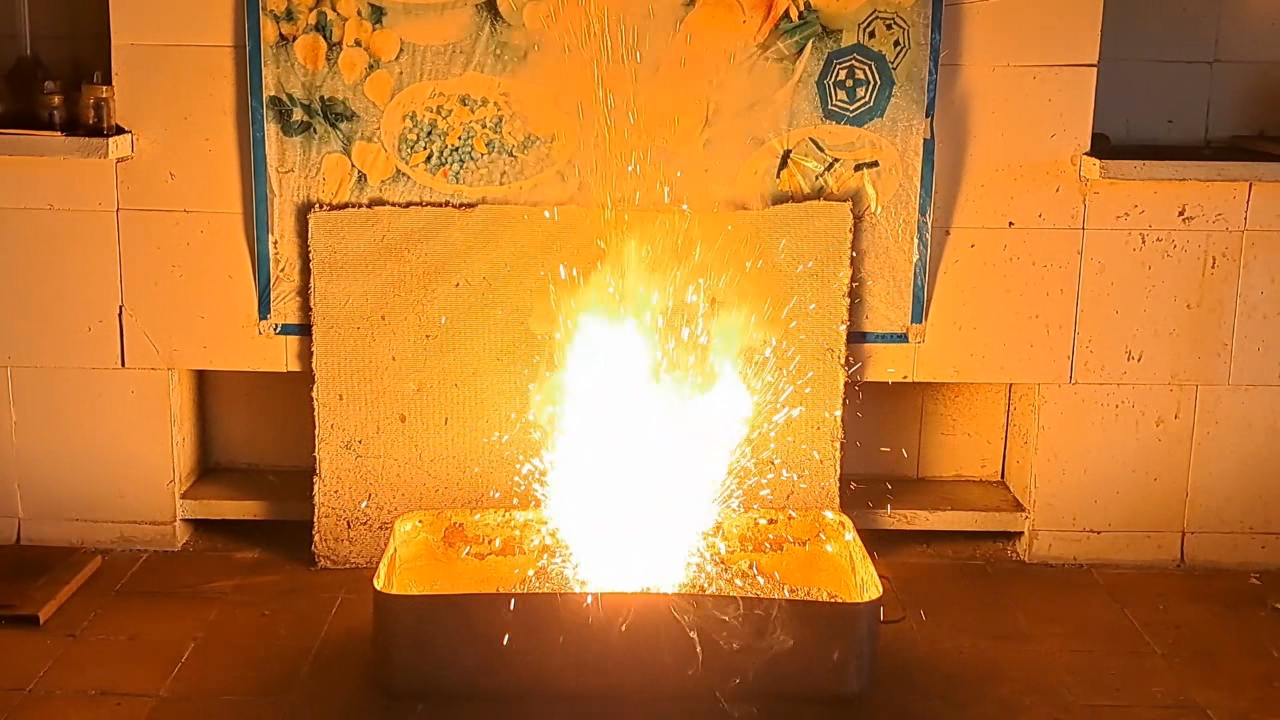
I decided to use aluminum waste to make thermite again, although previous experiments with it had produced mediocre results.
Горение термита: Fe3O4 + KNO3 + Al (отходы) - часть 15 I thoroughly mixed 25.9 g of aluminum waste, 57.2 g of iron (II, III) oxide (Fe3O4), and 15.1 g of potassium nitrate. I dried the mixture in a drying cabinet and poured it into an aluminum Pepsi-Cola can with the top cut off. After moderately compacting the thermite, I poured an incendiary mixture of 1 g of finely dispersed aluminum and 2.75 g of iron oxide (Fe3O4) on top of it. I then placed the can in an iron tray filled with dry sand. I directed the flame of a burner into the can. The thermite ignited. The aluminum walls of the can instantly disappeared. A bright yellow flame appeared, accompanied by hissing and the ejection of numerous sparks. White smoke was released during combustion. After the reaction had finished, a "lake" of molten iron and aluminum oxide formed on the sand, glowing brightly. There was so much white smoke that I asked a colleague to turn on the fume hood before the molten mixture had cooled down. Turning on the fume hood while the thermite is burning—or immediately after it finishes burning—is dangerous, as it can cause a fire. The hot mixture gradually cooled and eventually stopped glowing. However, a red glow was still visible on the phone screen. Apparently, the phone camera captured infrared radiation emitted by the mixture—radiation that is invisible to the naked eye. Once the mixture had cooled completely, a large black "drop" of porous iron and aluminum oxide had formed. The aluminum bottom of the can did not burn completely. Because of this seemingly minor detail, I was completely disappointed in the thermite made from waste. |

Combustion of Thermite: Fe3O4 + KNO3 + Al (waste) |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
|

|
|
|

|
|
|
|
|
|
|

|

|

|

|

|

|

|

|

|

|

|
|
Having noticed a mistake in the text, allocate it and press Ctrl-Enter
Combustion of Thermite: Iron(II, III) Oxide/Aluminum (Fe3O4/Al) - Part 16
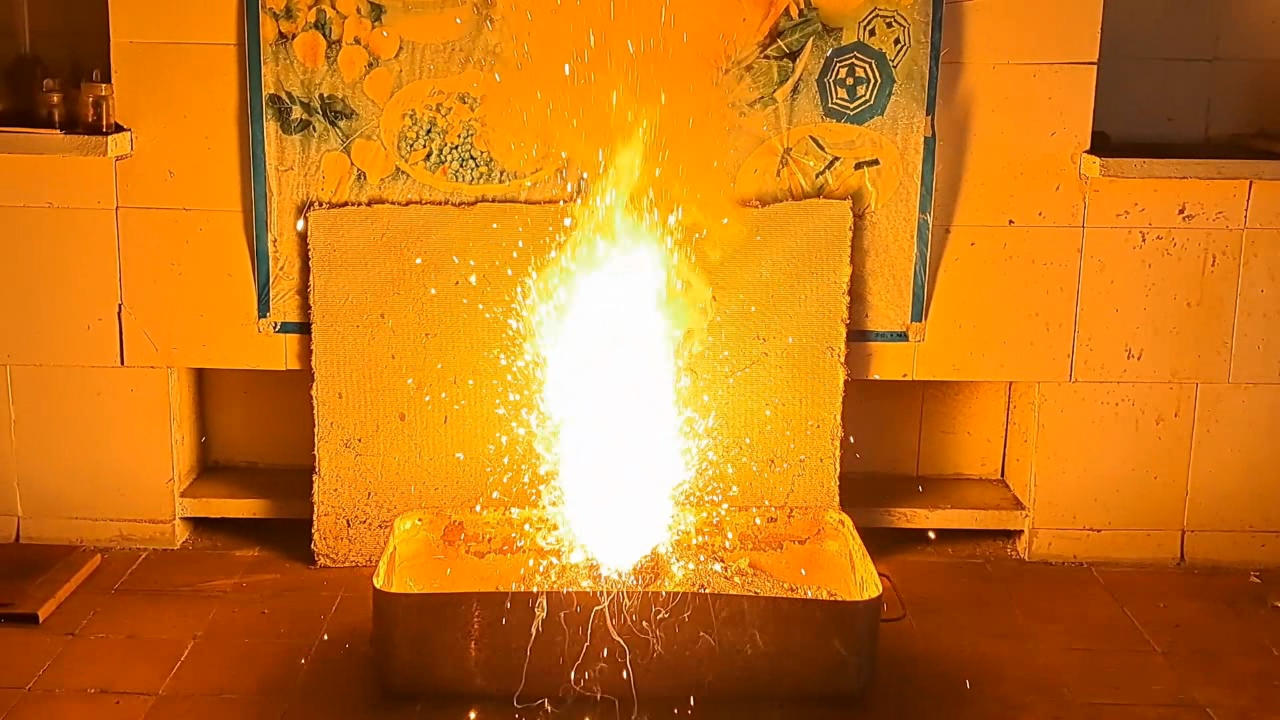
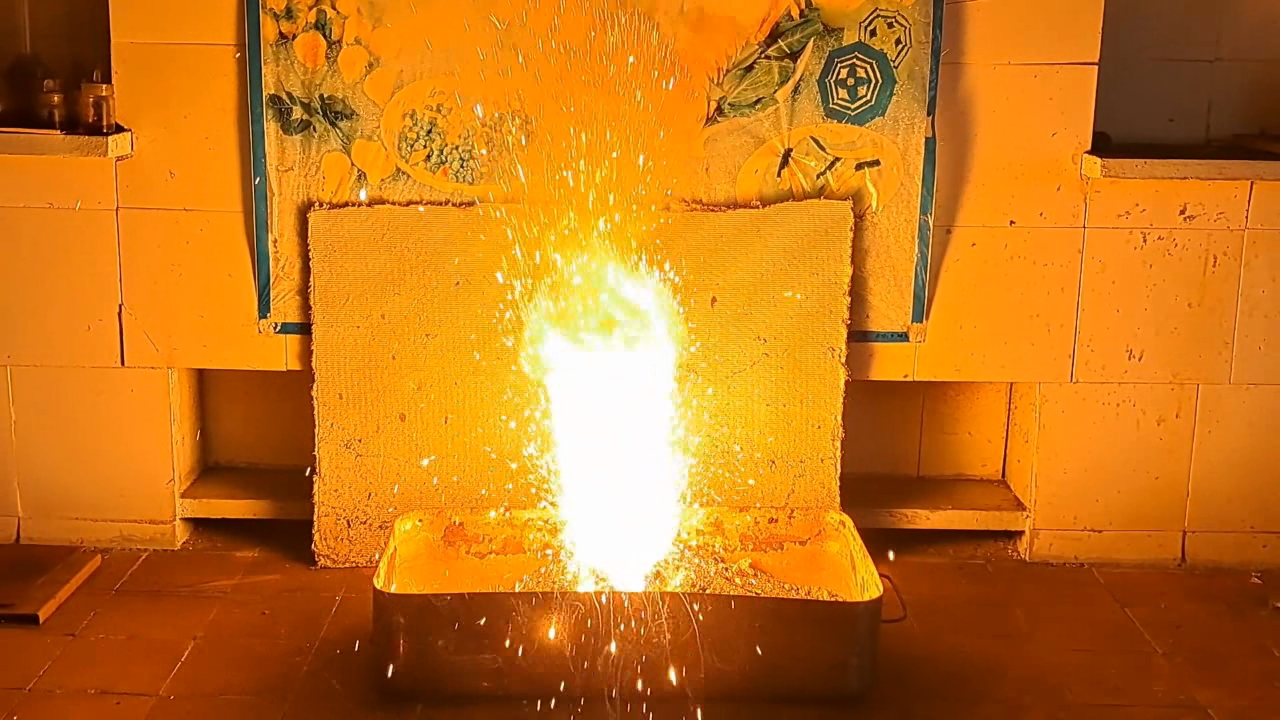
Since ceramic flower pots were destroyed during previous thermite combustions, I decided to return to using tin cans, this time coating their inner surfaces with clay. I prepared clay dough and applied a thick layer to the inner walls and bottom of the can. A hole was made in the bottom to allow the molten products to drain. The can was thoroughly dried in a drying cabinet at 220°C. I sealed the bottom hole with tape.
Горение термита: оксид железа (II, III)/алюминий (Al/Fe3O4) - часть 16 I prepared a mixture of 55 g of aluminum and 151.5 g of iron(II,III) oxide (Fe3O4), then poured the thermite into the can. Unlike in earlier experiments, I compacted the mixture as tightly as possible. Initially, I attempted to press the thermite down using a wooden pencil, but it proved unsuitable. I then used an iron pestle from a mortar, pressing down with full force and tapping it to ensure the thermite was thoroughly compacted. On top of the main thermite charge, I placed an incendiary mixture consisting of 1 g of finely powdered aluminum and 2.75 g of iron oxide (Fe3O4). The can was placed on an iron tripod, beneath which was an iron tray filled with dry sand, shaped into a mound. I had planned to cover the can with a tin lid immediately after ignition to limit heat loss, but I soon realized this would likely be impossible due to the violent nature of the combustion. I directed a gas burner flame at the thermite. A bright flash occurred: first the incendiary mixture ignited, followed by the main thermite charge. A yellow flame erupted from the can, accompanied by a shower of sparks. It was clearly too dangerous to attempt placing the lid on the can during the reaction. Compared to the previous experiment, there were fewer sparks, but they were larger and flew farther. In addition to the hissing sound, bubbling noises were heard, as if gas bubbles were bursting through a viscous liquid. As the combustion weakened, I attempted to cover the can again. At that moment, a stream of molten iron and aluminum oxide began flowing from the bottom hole. The blinding hot melt poured from the can for several seconds, flowing down the sand mound and gradually cooling. My mistake became clear: I should have formed a depression at the top of the sand mound (a sort of "volcano crater") so the melt would collect there rather than flowing down the sides. The leaked iron and the inside of the can glowed brightly as they slowly cooled. In this experiment, I successfully produced a powerful stream of molten iron and aluminum oxide flowing from the can, which is important for visually demonstrating thermite combustion to an audience. Compacting the thermite reduced the amount of air trapped between solid particles. During combustion, this trapped air expands, forming a flow that carries away heat and reaction material. The less air present, the less heat is lost. In my next experiment, I plan to cover the can with a lid before ignition to further minimize heat loss. |

Combustion of Thermite: Iron(II, III) Oxide/Aluminum (Fe3O4/Al) |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
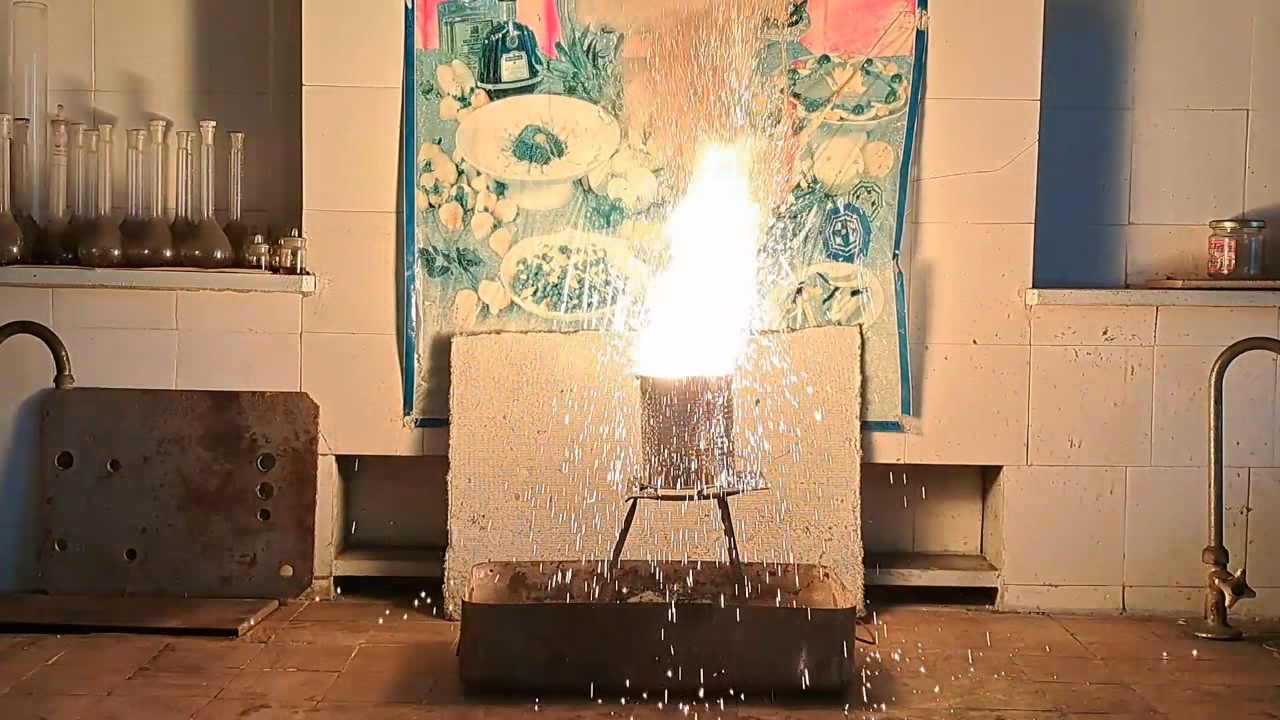
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|