Chemistry and Chemists № 2 2025
Journal of Chemists-Enthusiasts
| Content | Chemistry experiments - video | Physics experiments - video | Home Page - Chemistry and Chemists |
|
Chemistry and Chemists № 2 2025 Journal of Chemists-Enthusiasts |
Experiments with Thermite - pt.17, 18 Chemist |
|
Having noticed a mistake in the text, allocate it and press Ctrl-Enter
Combustion of Thermite: Iron(II, III) Oxide/Aluminum (Fe3O4/Al) - Part 17
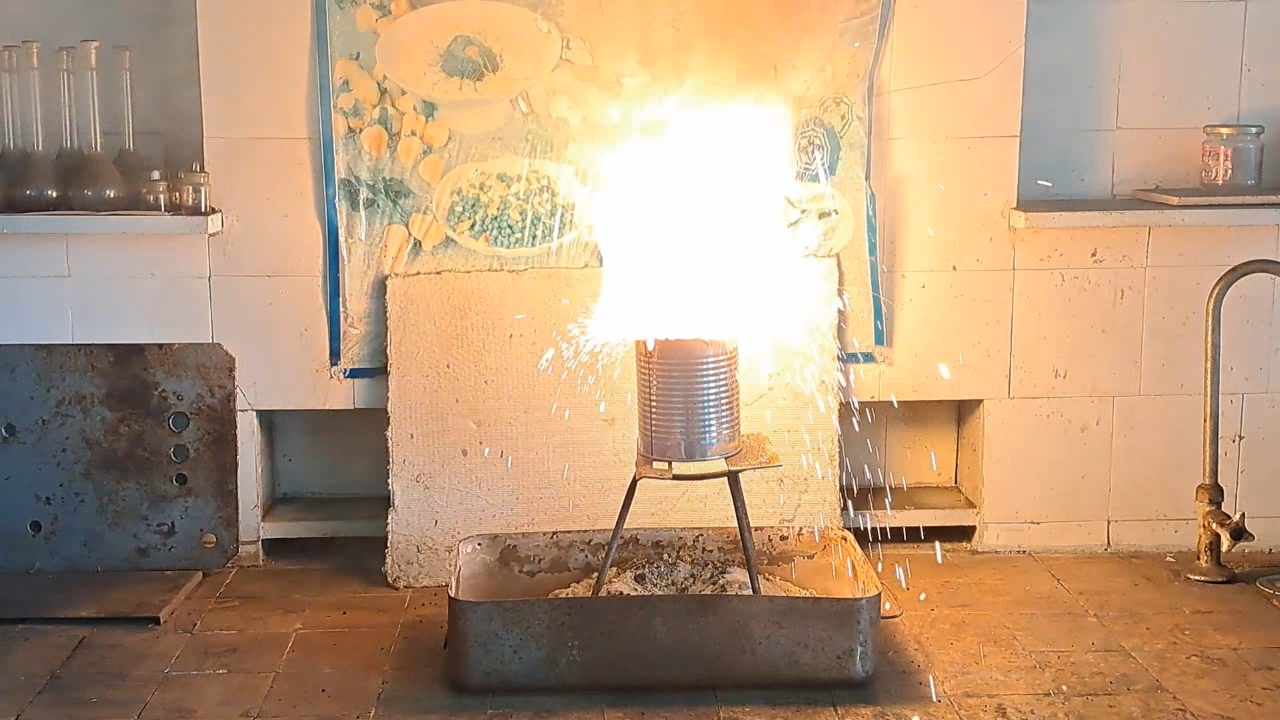
I reused the tin can from the previous experiment, making a hole in its bottom. The clay coating survived the thermite reaction. A layer of solidified melt formed on top of the clay.
Горение термита: оксид железа (II, III)/алюминий (Al/Fe3O4) - часть 17 Compared to the previous experiment, several changes were made. I increased the amount of thermite, mixing and pressing together 80 g of aluminum and 220 g of iron oxide (Fe3O4), for a total thermite mass of 300 g. This time, I poured black powder on top of the incendiary mixture and covered the can with a tin lid, leaving a narrow gap so that the flame from the burner could ignite the gunpowder. The gunpowder was expected to ignite the incendiary mixture, which would then ignite the bulk of the thermite. Upon seeing my preparations, a colleague remarked that the black powder would explode, throwing off the lid, while the incendiary mixture would likely fail to ignite. I directed the burner's flame into the gap under the lid. Everything happened just as my colleague predicted: the gunpowder ignited suddenly, the lid flew off, but the thermite did not catch fire. I put the lid back in place, this time widening the gap, and directed the flame straight at the incendiary mixture. It did not ignite immediately, but eventually caught fire, followed by ignition of the main thermite charge. A yellow flame shot out of the can, accompanied by smoke. Hissing, crackling, and bubbling sounds were heard. Despite the closed lid, large sparks flew in all directions, posing a fire risk to nearby objects. On the wall of the fume hood hung a polyethylene film with a still life image. In previous thermite experiments, several large holes had already appeared in the film. During this experiment, new holes were burned through it. Fortunately, the film did not catch fire. A fire extinguisher was kept nearby to quickly eliminate any potential sources of ignition. It was later discovered that the thermite flame had burned a large hole in the tin lid, meaning the lid did not protect against sparks. Forty seconds after ignition, a bright yellow stream of molten material flowed out of the hole at the bottom of the can. This time, I had prepared a depression in the sand beneath the can. The melt collected in this depression, forming a fiery "lake." Both the inside of the can and the molten lake glowed bright yellow as they gradually cooled. The solidified melt formed an ingot about the size of a matchbox. Some of the melt also solidified on the inner walls of the can. |

Combustion of Thermite: Iron(II, III) Oxide/Aluminum (Fe3O4/Al) |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
Having noticed a mistake in the text, allocate it and press Ctrl-Enter
Combustion of Thermite: Iron(II, III) Oxide/Aluminum (Fe3O4/Al) - Part 18
In most previous experiments, thermite combustion occurred in a heat-resistant vessel: a tin can, a clay pot, or a tin can coated with clay. This choice of container was intended to demonstrate the stream of molten iron and aluminum oxide that poured out of a hole in the bottom of the pot or can during the final stage of the reaction.
Горение термита: оксид железа (II, III)/алюминий (Al/Fe3O4) - часть 18 However, these heat-resistant vessels hindered direct observation of the thermite combustion itself. One could mainly see the flames and sparks erupting from the top of the vessel. The burning thermite was concealed by the opaque walls of the can or pot. This time, I used a cut-off aluminum beer can, the walls of which easily melt and burn during thermite combustion. I weighed and mixed 100 g of aluminum, 275 g of iron(II,III) oxide (Fe3O4), and 3.1 g of sulfur. The aluminum particles were more coarsely dispersed than in all previous experiments (particle size: 100-140 microns, grade "PA-4 / ПА-4"). I added sulfur for only one reason: I had a small amount contaminated with copper oxide that needed to be disposed of. I decided to use this sulfur as a binder. I poured the mixture into the aluminum can in portions, compacting it each time with blows from a large hammer (I struck it as hard as I could, trying not to destroy the thin can walls). On top of the pressed thermite, I poured a loose mixture of 1 g of finely dispersed aluminum and 2.75 g of iron oxide, which served as an incendiary charge. Conducting this experiment in my lab was out of the question—not only because of the large mass of thermite, but also because the container was not heat-resistant. When the thermite ignites, the aluminum can walls will melt and burn instantly (aluminum melts at 660°C). I chose a secluded spot in the yard and warned those nearby not to be alarmed by the "small" flame, smoke, and burning sounds. Just in case, I brought a fire extinguisher with me. I placed the can of thermite in an iron tray filled with dry sand, making a shallow depression under the can. I positioned the camera farther away than in previous experiments, out of concern that sparks might reach it. I directed a strong flame from a burner at the incendiary mixture. There was a bright flash. Combustion quickly engulfed the entire mass. A furious, wide, bright yellow flame erupted, scattering sparks with a loud hissing noise. Thick smoke formed. The sight was frankly frightening. On the downside, I had placed the camera too far away. Combustion lasted for about ten seconds. In previous experiments, smaller amounts of thermite in heat-resistant vessels burned for much longer. I took the smartphone in hand and came closer. Where the can of thermite had been, there was now a "lake" of molten iron and aluminum oxide, glowing bright yellow and still smoking. The thermite burned quickly, but the melt cooled slowly, as the mass was much greater than in earlier trials. Over time, a crust began to form on the surface of the fiery "lake," and molten material started to emerge from beneath the crust, occasionally emitting small sparks. The yellow glow gradually shifted to red and slowly faded. As it cooled, the mass cracked with a characteristic sound. I took a wooden stick and flipped over the solidified ingot. It had the shape of a one-sided magnifying lens and was about the size of an adult's palm. The mass was still glowing red. The wooden stick caught fire several times. |

Combustion of Thermite: Iron(II, III) Oxide/Aluminum (Fe3O4/Al) |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|