Chemistry and Chemists № 2 2025
Journal of Chemists-Enthusiasts
| Content | Chemistry experiments - video | Physics experiments - video | Home Page - Chemistry and Chemists |
|
Chemistry and Chemists № 2 2025 Journal of Chemists-Enthusiasts |
Identification of Copper(II) Oxide - pt.1, 2 Chemist |
|
Having noticed a mistake in the text, allocate it and press Ctrl-Enter
Copper(II) Oxide and Nitric Acid - Part 1
In my experiments with thermite, I used a mixture of copper(II) oxide and aluminum. The copper oxide came in an unusual form: small spheres. These copper oxide balls were hard and had to be crushed with hammer blows.
Идентификация оксида меди (II) Оксид меди (II) и азотная кислота - часть 1 Previously, I had worked with copper(II) oxide in the form of powder or small wire fragments. I had never seen copper oxide granules shaped like spheres. One reader of the article did not believe it was copper oxide and suggested that I had mistaken it for "pellets"—agglomerated iron ore formed into balls. Small particles of iron ore, once considered waste, are sintered into larger pieces suitable for further processing. Pellets are used in the smelting of steel and cast iron in blast, induction, and electric arc furnaces. They are widely used in the metallurgical and foundry industries, as well as in chemical production. According to the literature, iron ore pellets range in size from 1 mm to 30 mm, but I have seen pellets shaped like spheres about 10 mm or larger. In the past, such pellets could often be found along railroad tracks, having fallen from wagons during transport. The typical size of iron ore pellets was significantly larger than the copper(II) oxide spheres I used for thermite. However, to clearly distinguish copper(II) oxide from iron ore, I conducted an experiment. I placed several spherical granules of copper oxide in a glass and added about 20 ml of 68% nitric acid. Initially, the solution remained colorless, and a slight release of gas bubbles from the solid surface was observed. After a few minutes, the solution gradually began turning greenish-blue from the bottom. The color slowly intensified and spread upward, while a weak and barely noticeable gas release continued. I left the glass over the weekend. After two days, the solution had turned deep blue, but the copper oxide spheres had not dissolved. This confirmed that the spheres were indeed copper(II) oxide, which reacted slowly with nitric acid to form a blue copper nitrate solution: CuO + 2HNO3 = Cu(NO3)2 + H2O Unlike copper(II) nitrate, iron(III) nitrate forms a yellow or brown solution. I covered the glass with a Petri dish and left it unattended for nearly a month while continuing my thermite experiments. |

Copper(II) Oxide |

|

|

Iron ore pellets |

Iron ore pellets |

Iron ore pellets |

Copper(II) Oxide and Nitric Acid |

|
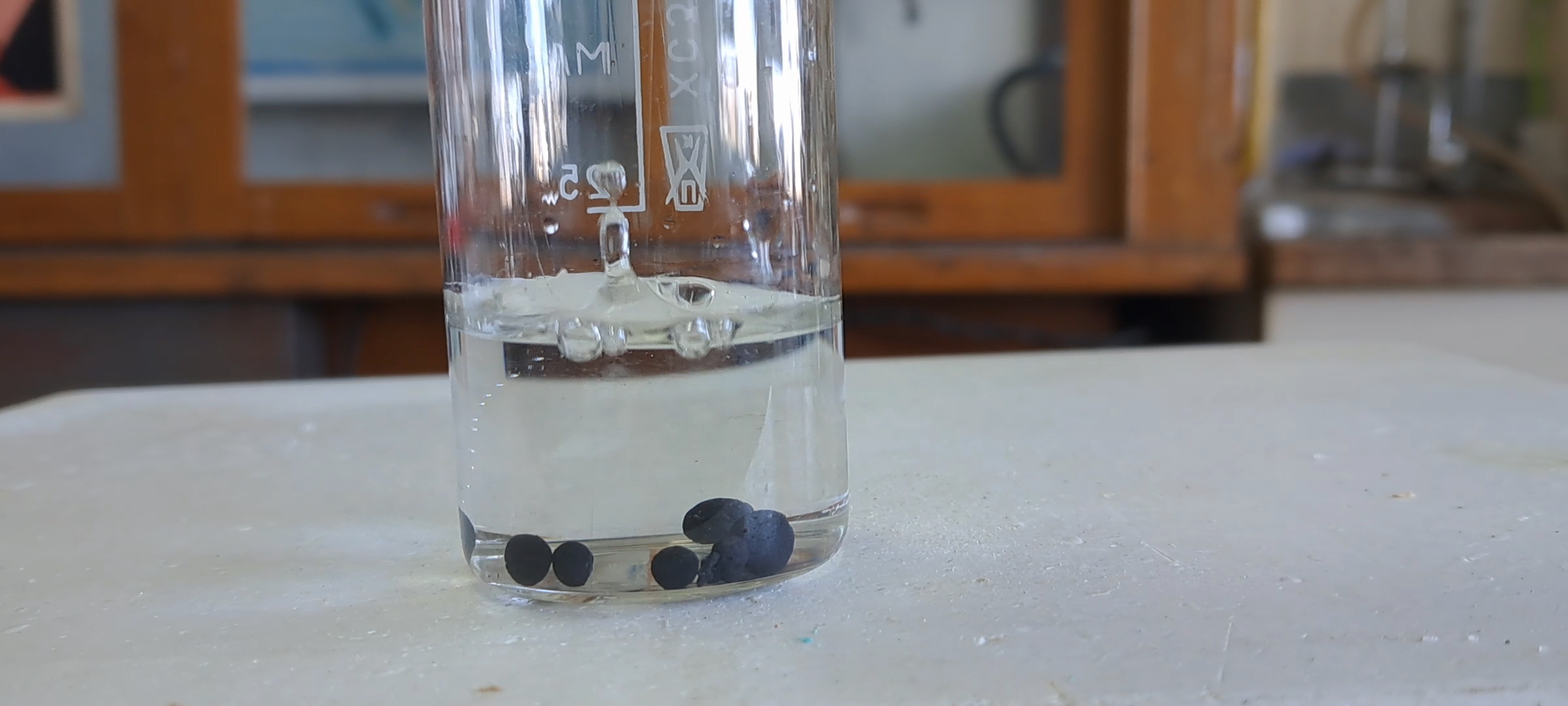
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

On the next day |

|
|
Having noticed a mistake in the text, allocate it and press Ctrl-Enter
Copper(II) Oxide, Nitric Acid and Ammonia - Part 2
I was busy with some labor-intensive work, so I left the glass containing copper oxide and nitric acid unattended. Only a month later, I had the opportunity to continue the experiment. By that time, the glass contained an intensely blue solution of copper nitrate; all the copper(II) oxide had dissolved.
Оксид меди(II), азотная кислота и аммиак - часть 2 A characteristic property of divalent copper ions is the formation of [Cu(NH3)4]2+, a dark blue complex with ammonia. I drew some of the blue copper nitrate solution into a pipette and added it to a concentrated solution of ammonia. The copper nitrate solution sank to the bottom and turned dark blue, indicating the formation of the [Cu(NH3)4]2+ cation. White smoke of ammonium nitrate formed above the solutions of ammonia and copper nitrate. This is explained by the presence of excess nitric acid in the copper nitrate solution. The nitric acid reacted with ammonia not only in the liquid phase but also in the gas phase, forming solid particles of ammonium nitrate. Cu2+ + 4NH3 + 2OH- = [Cu(NH3)4](OH)2 HNO3 + NH3 = NH4NO3 When I added further portions of the copper nitrate solution to the glass containing concentrated ammonia, a hissing sound was heard, and thick white smoke formed. The more copper nitrate I added, the darker the solution became. Eventually, it turned almost black. This darkening is due to the increasing concentration of the intensely colored [Cu(NH3)4]2+ cation. However, after adding another portion of the copper nitrate solution, the ammonia solution did not become darker. Instead, it turned into a light blue suspension. Upon adding yet another portion of the copper nitrate, the suspension transformed back into a transparent blue solution, as the solid phase dissolved. This behavior can be explained by the fact that the copper nitrate solution also contained nitric acid, which reacted with ammonia and with the [Cu(NH3)4](OH)2 complex: [Cu(NH3)4](OH)2 + 4H+ = Cu(OH)2(solid) + 4NH4+ Cu(OH)2 + 2H+ = Cu2+ + 2H2O Initially, copper hydroxide (Cu(OH)2) precipitated, but it later dissolved in the excess acid to form copper nitrate (Cu(NO3)2). So, by adding a solution containing copper nitrate and nitric acid to ammonia, we observed the transformation of copper(II) nitrate into the ammonia complex, then into copper hydroxide, and finally back into copper nitrate. In conclusion, I would like to note that describing the colors of copper compounds proved difficult. In photos and videos, the colors of copper nitrate and the copper-ammonia complex [Cu(NH3)4]2+ appear similar due to color distortion by the camera. In reality, the color of the copper nitrate solution was more like "sky blue", while the copper-ammonia complex was "navy blue". It is also interesting that in Ukrainian, the colors of the cations [Cu(H2O)4]2+ (light sky blue) and [Cu(NH3)4]2+ (navy blue) correspond to two distinct colors of the rainbow, whereas in English, both are typically referred to as simply "blue". |

Copper(II) Oxide, Nitric Acid and Ammonia |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|