Chemistry and Chemists № 2 2025
Journal of Chemists-Enthusiasts
| Content | Chemistry experiments - video | Physics experiments - video | Home Page - Chemistry and Chemists |
|
Chemistry and Chemists № 2 2025 Journal of Chemists-Enthusiasts |
Organic Peroxides and Concentrated Sulfuric Acid - pt. 1, 2 Chemist |
|
Having noticed a mistake in the text, allocate it and press Ctrl-Enter
HMTD (Hexamethylene Triperoxide Diamine) and Concentrated Sulfuric Acid: Does an Explosive Reaction Occur? - Part 1
Hexamethylene triperoxide diamine (HMTD) is an unstable organic peroxide that can explode due to impact, friction, fire, or static discharges. Acetone peroxide, another common organic peroxide, exhibits similar behavior to HMTD.
Органические перекиси и концентрированная серная кислота ГМТД (гексаметилентрипероксиддиамин) и концентрированная серная кислота: наблюдаться ли реакция с взрывом? - часть 1 In addition, HMTD and acetone peroxide can explode upon contact with certain compounds, with concentrated sulfuric acid often cited in the literature as one of them. This information is not only found on Wikipedia but also in reputable scientific monographs, lending it a degree of credibility. When a colleague suggested that I conduct this experiment, I was initially hesitant. Why drip sulfuric acid onto HMTD when simply striking it with a hammer or exposing it to a flame would be enough to trigger an explosion? On the other hand, consider this from the perspective of an audience. When viewers see a substance struck with a hammer or ignited, they anticipate a dramatic reaction. However, dripping liquid from a pipette onto a white powder will surprise the audience, as no one expects an explosion or flash in such a scenario. My colleague was right: an explosion triggered by a drop of liquid could serve as an effective chemical demonstration. This is particularly impactful if you tell the audience that the liquid in the pipette is water and the powder is something innocuous like flour. Unfortunately, I no longer had any acetone peroxide, as I had given my stock to a chemist working with polymers. Since storing organic peroxides is dangerous, I hadn't kept even a small amount. Lacking the inclination to re-synthesize acetone peroxide, I prepared a small quantity of HMTD instead. The synthesis is simple, and the starting materials are readily available. How was the experiment conducted? First, I weighed 0.1 g of HMTD in a plastic cap and poured some concentrated sulfuric acid into a separate glass. Then came the question: how to proceed safely? Adding sulfuric acid to HMTD could result in a violent reaction, scattering acid onto my clothing, face, and smartphone (which I use to record videos). Sulfuric acid readily damages clothing, footwear, and electronic devices. By the way, this effect is caused not only by sulfuric acid. My previous camera, for instance, suffered multiple breakdowns after being exposed to concentrated nitric acid during experiments. To protect my smartphone, I placed a sheet of plexiglass between the HMTD and the camera, although this compromised the video quality. I wore safety goggles and rubber gloves to shield myself from potential splashes. Additionally, I put on old clothes and shoes, as it would not have mattered if they were damaged by the acid. With everything ready, I was eager to see whether an explosion would occur. I added a drop of sulfuric acid to the HMTD, but nothing happened. I waited a few seconds and added a few more drops - still no explosion, flash, or any other visible sign of a reaction. After two minutes, I added about half a milliliter of the acid. Four minutes later, I gently shook the cap's contents with tweezers, but no reaction occurred. The low temperature in the laboratory (4°C) might have been the culprit, as cooling slows most chemical reactions. I remembered that adding water to concentrated sulfuric acid generates significant heat, often leading to violent boiling. To induce heating, I added four drops of water to the cap. After half a minute, I added a few more drops. Soon, the mixture began to hiss, releasing a white aerosol. A small flash occurred, leaving behind a brown liquid. However, the mixture did not ignite fully or explode because I had added too much liquid (sulfuric acid and water) to the HMTD. I repeated the experiment with 0.1 g of HMTD on a polyethylene cap. This time, I added five drops of sulfuric acid, followed by two drops of water half a minute later. For nearly a minute, nothing significant occurred. Just as I reached to add another drop of water, a strong yellow flash erupted, accompanied by the characteristic sound of HMTD combustion. A thick brown residue remained in the cap. Interestingly, the polyethylene cap was undamaged, despite its lack of thermal stability. Had I used more HMTD, the flash would have been stronger, potentially escalating to an explosion. Thus, the claim that concentrated sulfuric acid can cause HMTD to explode has been experimentally confirmed. |

HMTD (Hexamethylene Triperoxide Diamine) and Concentrated Sulfuric Acid |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
Having noticed a mistake in the text, allocate it and press Ctrl-Enter
Acetone Peroxide and Concentrated Sulfuric Acid - Part 2
In the previous experiment, HMTD (hexamethylene triperoxide diamine) ignited upon contact with concentrated sulfuric acid. However, some issues arose: water had to be added to initiate the flash. It seemed logical to conduct a similar experiment with acetone peroxide. Perhaps, with this substance, an explosion or flash would occur more easily than with HMTD.
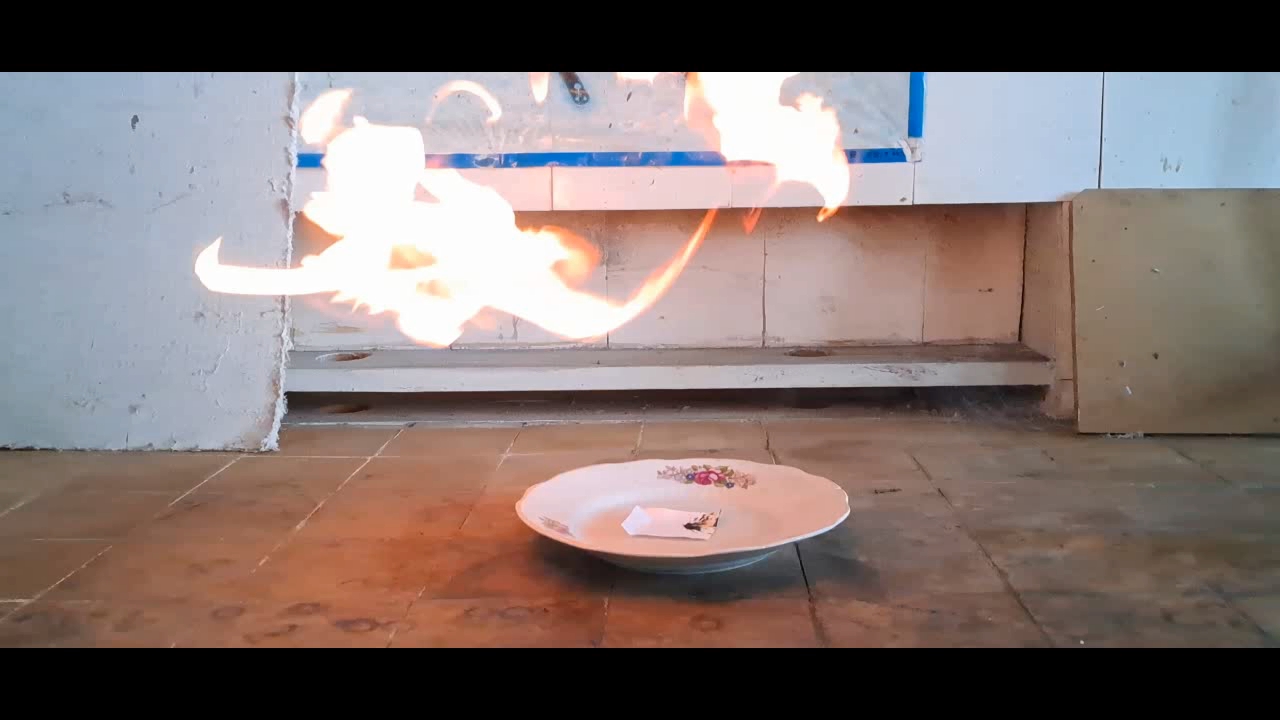
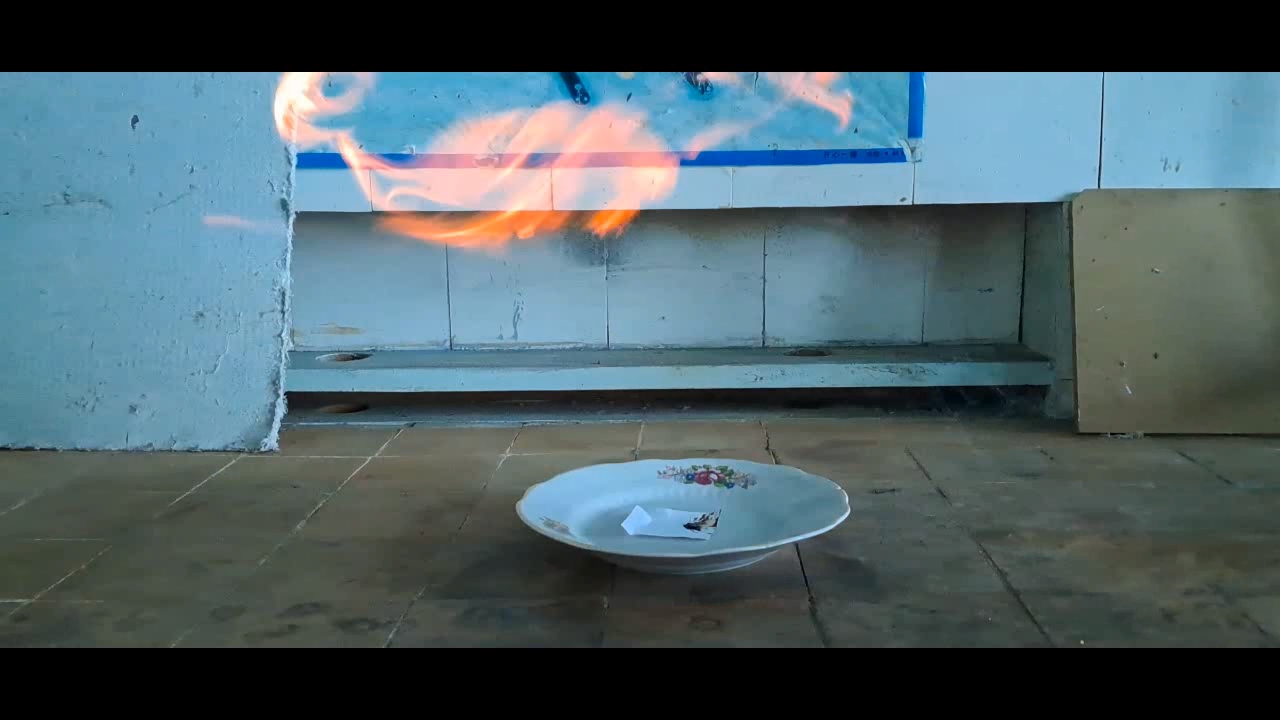
Перекись ацетона и концентрированная серная кислота However, there were also reasons against conducting this experiment. Acetone is the starting material for synthesizing acetone peroxide, and I had little left. I also did not want to repeat this rather routine synthesis. Finally, I was unsure whether acetone peroxide would perform better than HMTD in this case. Given these factors, I hesitated to proceed. Suddenly, my doubts disappeared. My colleagues had used only part of the benzoyl peroxide and acetone peroxide I had given them earlier, leaving me with some remaining material to use. I weighed 0.23 g of acetone peroxide and placed it on a piece of paper, forming a small pile. I then made a slight depression in the center for the sulfuric acid. The paper was set on a porcelain plate. Incidentally, after my predecessors, little chemical glassware remained in the lab, but there were plenty of porcelain kitchen items - plates, cups, spoons, and so on. Therefore, if the plate were to break during the experiment, it would not be a significant loss. To protect the camera from acid splashes, I used a sheet of plexiglass. Since the weather had warmed up, the temperature in the lab was now 8°C. For comparison, during the HMTD experiments, the room had been slightly colder (4°C). As a reminder, lowering the temperature slows down most chemical reactions, which means that the colder the room, the less likely acetone peroxide is to explode or ignite upon contact with sulfuric acid. Let's begin the experiment. I dropped concentrated sulfuric acid onto the acetone peroxide. The drop rolled down onto the paper, which immediately charred. A hissing sound followed as gas bubbles and white aerosol were released. Would I really have to wait long for the reaction to start? However, within seconds, a yellow flash occurred, accompanied by a loud pop. The paper was not visibly changed by the flame but turned black where the acid had made contact. I repeated the experiment with 0.24 g of acetone peroxide. This time, I placed the cellphone further away, allowing me to remove the protective screen. As soon as I added the sulfuric acid, the flash happened so quickly that I did not have time to move my hand away. Splashes of concentrated sulfuric acid hit my face. The pop was louder this time. Once again, the paper charred where the acid had touched it, but the rest remained unchanged. Thus, this experiment with acetone peroxide proved to be much more efficient than the one with HMTD. |

Acetone Peroxide and Concentrated Sulfuric Acid |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
|

|

|