Chemistry and Chemists № 2 2025
Journal of Chemists-Enthusiasts
| Content | Chemistry experiments - video | Physics experiments - video | Home Page - Chemistry and Chemists |
|
Chemistry and Chemists № 2 2025 Journal of Chemists-Enthusiasts |
What Was Used to Falsify Acetone? - pt.1, 2 Chemist |
|
Having noticed a mistake in the text, allocate it and press Ctrl-Enter
Falsified Acetone and Sodium Hydroxide Solution - Part 1
After a long absence from stores, acetone - whose sale has been prohibited in our country for a long time - has finally reappeared. I was delighted, as acetone is an important solvent and reagent for many chemical applications. However, my excitement was short-lived. It turned out that the solvent being sold as "acetone" contained very little actual acetone (hereinafter this liquid is referred to as "falsified acetone").
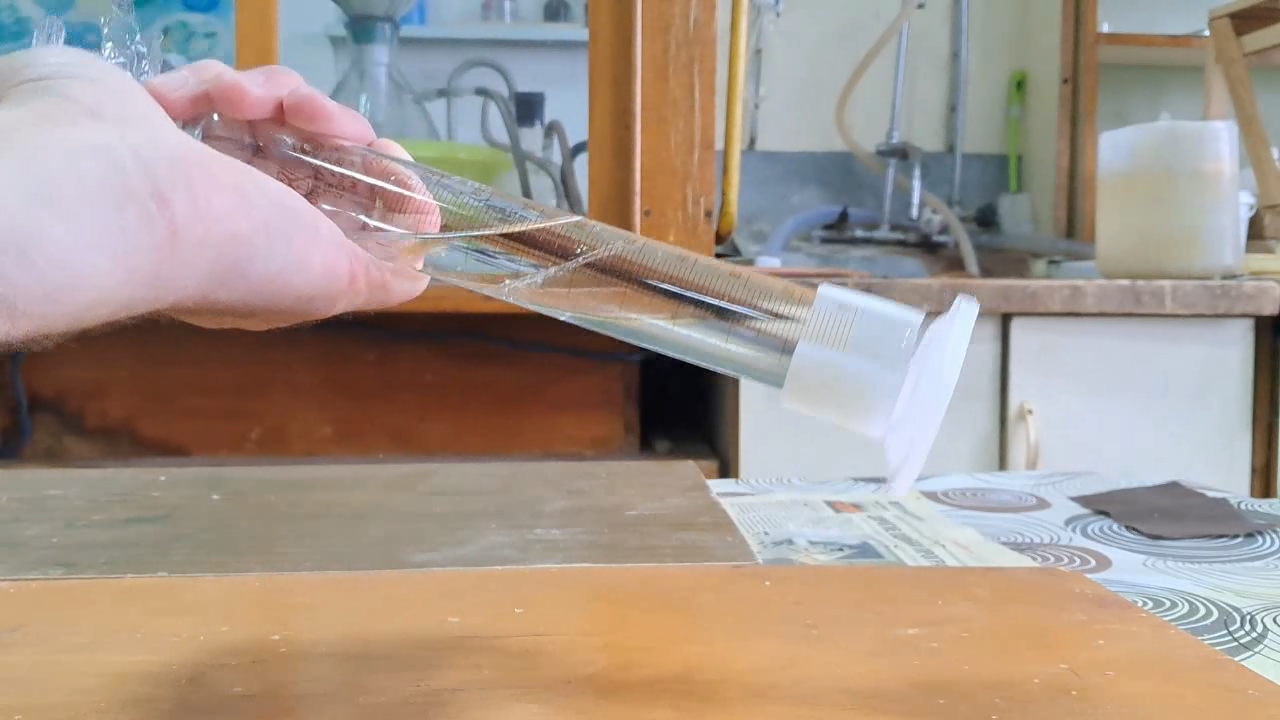
Что было использовано для фальсификации ацетона? Фальсифицированный ацетон и раствор едкого натра - часть 1 While pure acetone is infinitely soluble in water, falsified acetone was only partially soluble (approximately 1/5 to 1/4 of its volume dissolved). The remaining portion formed a separate layer on the surface of the water, emitting a strong smell of petroleum products. It was logical to assume that technical-grade acetone had been diluted with petroleum products and that this mixture was being sold as acetone. The reason behind this could be that the ban on acetone sales remains in effect, while the mentioned "substitute" is not subject to the same restrictions. One reader suggested that the acetone being sold was heavily diluted with methyl acetate. According to him, the mixture contained very little actual acetone - just enough to "create the smell of acetone." I have not personally worked with methyl acetate, but according to the literature, this substance has a fruity odor. However, the falsified acetone I purchased did not smell fruity at all; instead, it had a strong odor of acetone mixed with petroleum products. There is no contradiction between these observations, as different manufacturers may falsify acetone in different ways. Some might add petroleum products, while others may dilute it with methyl acetate or any other cheap, readily available solvent. Some manufacturers might even mix whatever they have on hand and sell the resulting liquid as "acetone." To identify petroleum products and esters in the mixture, chromatographic analysis would be required, which necessitates specialized equipment (such as a gas or liquid chromatograph). Since smell alone is not a strict criterion for identifying substances in mixtures, a simple chemical test can help distinguish esters from petroleum products. Under alkaline conditions, esters undergo hydrolysis. In the case of methyl acetate, hydrolysis produces methyl alcohol and sodium acetate, both of which are highly soluble in water. Petroleum products, on the other hand, do not dissolve in either water or aqueous alkali solutions. To perform this test, pour a measured volume of falsified acetone into a graduated cylinder, then add an excess of sodium hydroxide (or potassium hydroxide) solution. Seal the cylinder tightly to prevent evaporation, shake it thoroughly, and allow the liquids to separate. Measure the volume of the upper organic phase. Repeat the shaking and separation process periodically, recording the volume of the organic phase each time. If acetone is diluted with petroleum products, shaking it with a sodium hydroxide solution will initially cause the volume of the organic phase to decrease, as some of the acetone transitions into the aqueous phase. However, with further shaking, the volume of the organic phase will remain unchanged, since hydrocarbons do not dissolve in water. If acetone is diluted with methyl acetate (or similar esters), the volume of the organic phase will steadily decrease over time until it disappears completely due to the complete hydrolysis of the ester. A combination of both scenarios is also possible - where acetone is diluted with both hydrocarbons and esters. In this case, acetone will first dissolve into the water, then the volume of the organic phase will gradually decrease as the esters undergo hydrolysis. Once hydrolysis is complete, the volume of the organic phase will stop changing, leaving only the undissolved hydrocarbons. Experiment
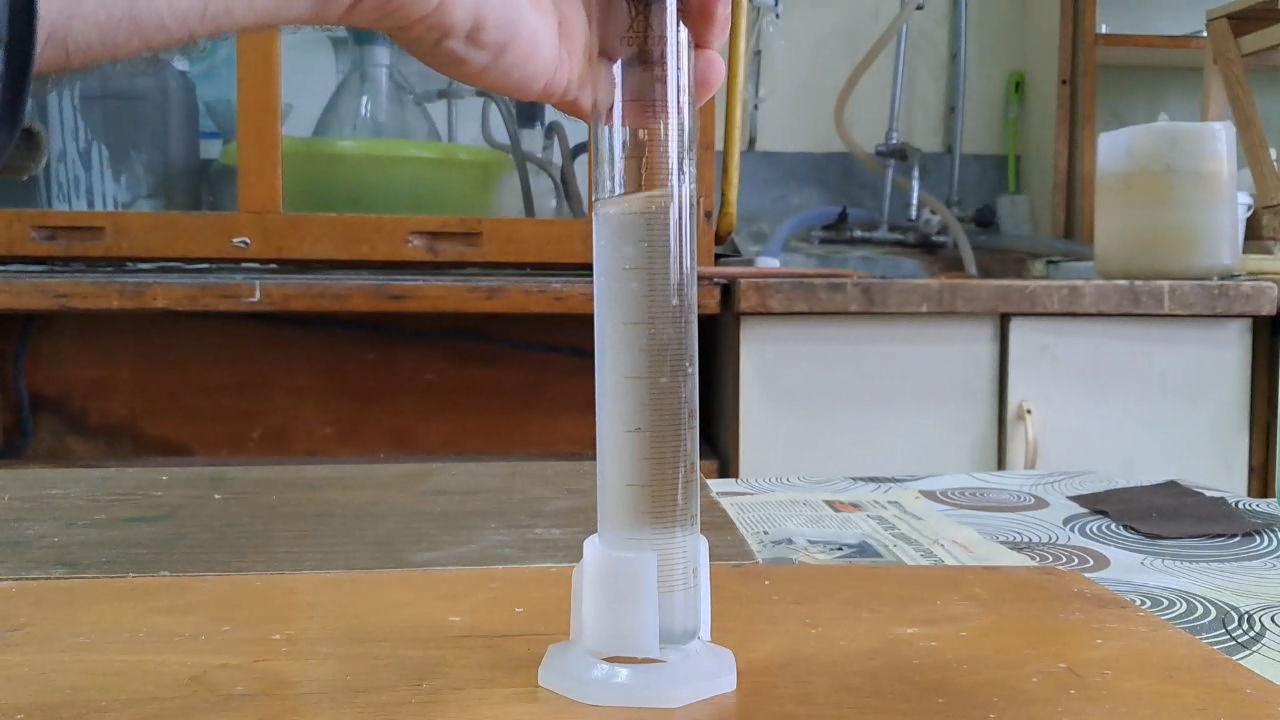
I dissolved 17.6 g of sodium hydroxide in 50 ml of water, preparing a 26% solution. Then, I poured 28 ml of falsified acetone into a graduated cylinder and added the sodium hydroxide solution. After sealing the cylinder tightly, I shook it vigorously multiple times. This resulted in the formation of an emulsion, which gradually separated into two distinct layers: a lower layer of sodium hydroxide solution and an upper layer of a lighter organic solvent. The volume of the organic layer was measured at 25 ml.
The reduction in the volume of the organic phase after shaking with the sodium hydroxide solution was: 28 - 25 = 3 ml (11%) Initially, I did not realize that the decrease in the organic phase volume was actually smaller than when shaking the falsified acetone with distilled water alone, where the reduction was approximately 20-25%. I left the graduated cylinder for 12 hours. The volume of the liquid phase did not change during this time. Since the majority of the falsified acetone did not dissolve in excess sodium hydroxide, methyl acetate (or other esters) were not used in its production. Instead, the results suggest that this batch of falsified acetone was most likely diluted with hydrocarbons (petroleum products). |

Falsified Acetone and Sodium Hydroxide Solution |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
Having noticed a mistake in the text, allocate it and press Ctrl-Enter
Ethyl Acetate and Sodium Hydroxide Solution - Part 2
In the previous experiment, falsified acetone was treated with a sodium hydroxide solution to test the assumption that the counterfeit acetone was primarily composed of methyl acetate (methyl ester of acetic acid). Upon shaking with the alkali solution, only a slight decrease in the volume of the organic phase was observed. This indicated that the falsified acetone did not contain significant amounts of methyl acetate or other esters.
Этилацетат и раствор гидроксида натрия - часть 2 To visualize this result, a comparison experiment was needed: treating pure methyl acetate with an alkali solution. Unfortunately, methyl acetate was not available. At the time of writing this article, I had never worked with methyl acetate or even seen this compound. However, this was not an obstacle, as ethyl acetate (ethyl ester of acetic acid) was readily available. Ethyl acetate is an analogue of methyl acetate - both undergo hydrolysis in acidic or alkaline solutions. I was unsure how quickly the hydrolysis would occur. In the literature, I found a description of a laboratory experiment for students in which ethyl acetate was dissolved in alcohol and then added to an alkali solution. The mixture was maintained at a specific temperature, and the concentration of the alkali was analyzed by titration at set intervals. By knowing the initial alkali concentration and tracking its decrease over time, the reaction rate constant was determined. In our case, a quantitative chemical analysis was unnecessary. The goal was simpler: to demonstrate that ethyl acetate undergoes hydrolysis when treated with alkali, with the reaction products dissolving in the aqueous phase. As a result, the organic solvent layer completely disappears.This procedure allows us to distinguish between acetone falsified with methyl acetate and acetone diluted with petroleum products. Experiment
After the previous experiment, a sodium hydroxide solution remained, with a layer of the organic solvent floating above it. I transferred the liquids into a separatory funnel and drained the lower alkaline layer into a beaker for reuse in this experiment.
I measured 30 mL of ethyl acetate in a graduated cylinder and added the sodium hydroxide solution. Initially, an emulsion formed in the aqueous phase. It was noticeable that this emulsion was more transparent (less turbid) than the one observed in the experiment with falsified acetone. This suggested that the refractive indices of ethyl acetate and the sodium hydroxide solution were similar. The emulsion quickly separated into two layers: the sodium hydroxide solution at the bottom and ethyl acetate on top. I attempted to mix the liquids with a glass rod, but this was ineffective. I then sealed the cylinder tightly with a stopper and shook it vigorously, forming an emulsion. When left to stand, the liquids gradually separated again, as ethyl acetate droplets coalesced and rose to form the upper layer. After repeated shaking, the emulsion initially formed but then broke down. At the time of the experiment, the laboratory temperature was 5°C. However, during shaking, the cylinder became noticeably warm. This indicated that ethyl acetate was hydrolyzing, forming acetic acid. The acetic acid then reacted with sodium hydroxide, releasing heat, as a neutralization reaction between an acid and a base is exothermic. CH3COOC2H5 + H2O <=> CH3COOH + C2H5OH CH3COOH + NaOH = CH3COONa + H2O + Q Heating was not the only change I observed. Soon, I noticed a curious phenomenon: during the breaking of the emulsion, gas bubbles formed. However, the release of gas stopped once the emulsion fully separated into two distinct liquid layers. A probable explanation for this phenomenon is the local overheating of ethyl acetate, causing it to boil. As is well known, the rate of heterogeneous reactions increases with a greater contact surface between the reactants. When the liquids were shaken, an emulsion of ethyl acetate in an aqueous sodium hydroxide solution formed, and this process led to a significant increase in the contact surface between the phases. Consequently, the reaction rate increased as well. This acceleration led to greater heat release, which in turn caused localized boiling of ethyl acetate. Once the ethyl acetate droplets coalesced into a continuous layer, the contact surface decreased, slowing the reaction, reducing heat generation, and stopping the boiling. As the shaking and separation cycles continued, the volume of ethyl acetate gradually decreased to 10 mL. To speed up the hydrolysis, I added 5.6 g of solid sodium hydroxide to the cylinder, sealed it, and shook the contents. This time, after shaking, the emulsion completely converted into a clear solution - no separate ethyl acetate layer remained when the liquid was left to stand. Thus, all the ethyl acetate had hydrolyzed into sodium acetate and ethanol, both of which dissolved in the aqueous phase. If acetone had been adulterated with ethyl acetate (or methyl acetate), such a falsified product would also completely dissolve in an alkali solution. |

Ethyl Acetate and Sodium Hydroxide Solution |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|

|
|

|

|

|

|

|

|

|

|

|

|

|

|

|