Chemistry and Chemists № 2 2025
Journal of Chemists-Enthusiasts
| Content | Chemistry experiments - video | Physics experiments - video | Home Page - Chemistry and Chemists |
|
Chemistry and Chemists № 2 2025 Journal of Chemists-Enthusiasts |
Fire on Hand: The Combustion of Acetone Peroxide Chemist |
|
Having noticed a mistake in the text, allocate it and press Ctrl-Enter
Acetone peroxide is an extremely hazardous substance, yet it has an unusual property: small amounts can undergo combustion on the palm of your hand without causing injury. Your skin is truly on fire - but the flame does not burn you!
However, if the same amount of acetone peroxide were to explode in your hand, traumatic amputation of your fingers would be possible, especially if you were holding the substance tightly. Loss of vision, damage to the eardrums, and other severe injuries could also occur. Therefore, before describing the experiment, I must say a few words about safety. Question: How can this experiment be conducted safely? Answer: There is no safe way to work with organic peroxides. Acetone peroxide and similar compounds can explode unpredictably, without any apparent cause. For instance, a static discharge from an electrified sweater could be enough to trigger detonation. A colleague of mine lost his fingers when a test tube containing several grams of acetone peroxide exploded in his hand. The likely cause was a static discharge. Another colleague once left a beaker, in which he had precipitated acetone peroxide, unattended on a windowsill. The window blew open, the beaker tipped over, and the liquid and precipitate spilled. By the time he returned, the liquid had evaporated, leaving the precipitate dry. When he attempted to scrape the dried substance off the windowsill, an explosion occurred. The blast shattered the window, caused a severe concussion, and led to long-term mental health issues. Strict adherence to safety protocols significantly reduces the risk of accidents when working with organic peroxides. However, no precautions can eliminate the danger entirely. When handling such substances, you are always risking your health - and that of those around you. I am aware that many will ignore my warnings. So, I will not attempt to dissuade anyone from experimenting with organic peroxides. But, for God's sake, never forget what you are dealing with! Even for a second! You might perform this experiment a hundred times without incident, but on the hundred-and-first, you could lose your eyes or fingers. The Experiment
Why did I conduct this experiment? Two physicists visited my lab, and I wanted to demonstrate a few chemical reactions, including the "fire on hand" phenomenon. To prepare for this, I first synthesized several grams of acetone peroxide.
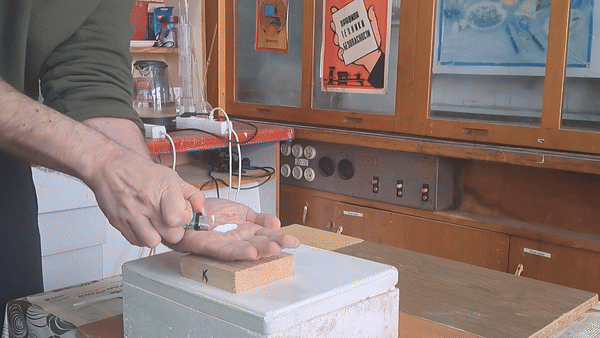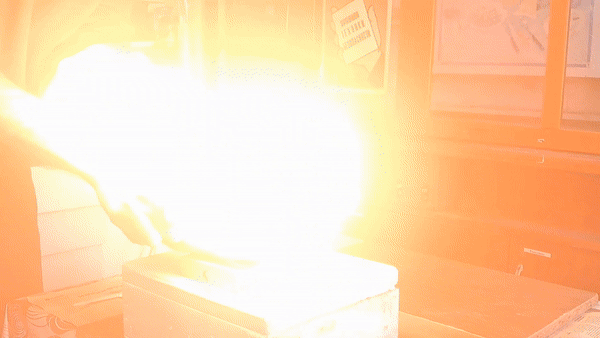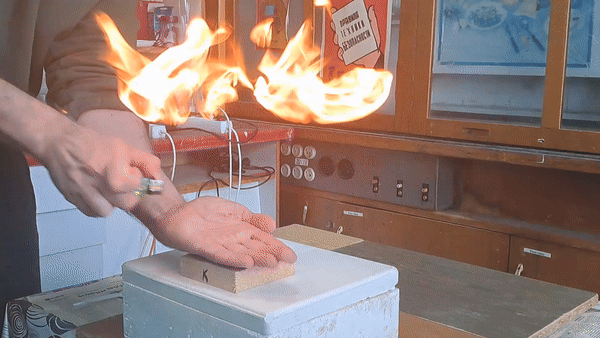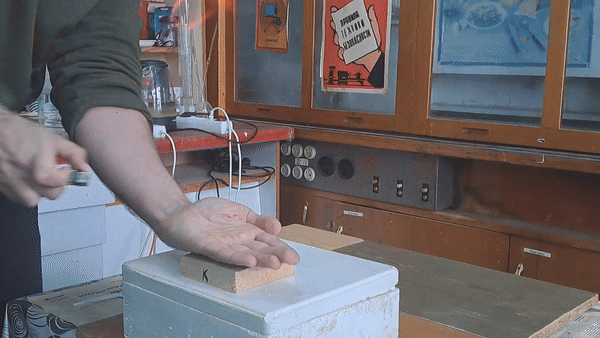
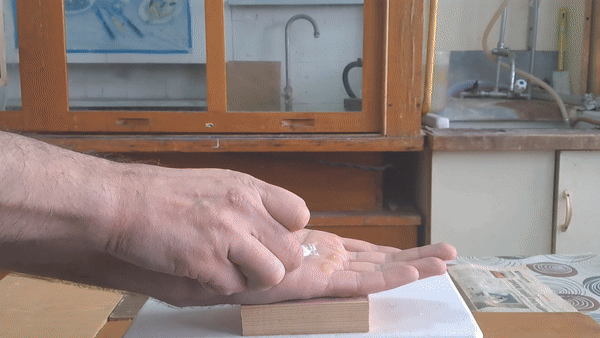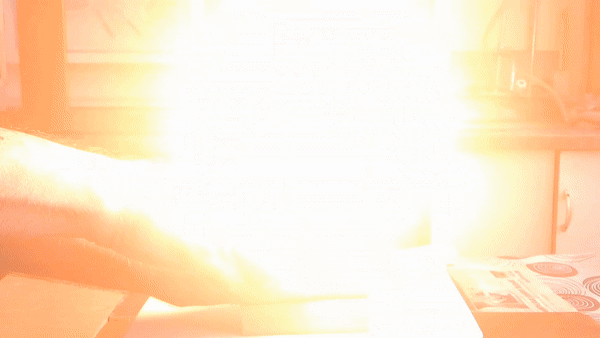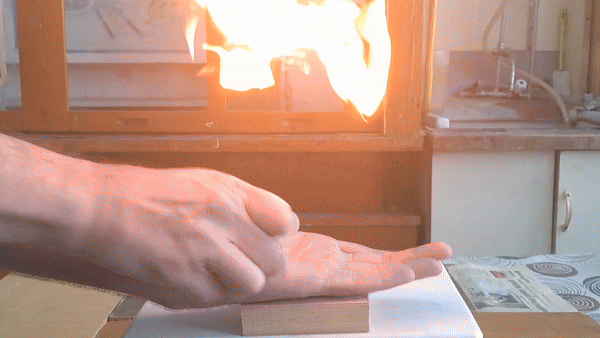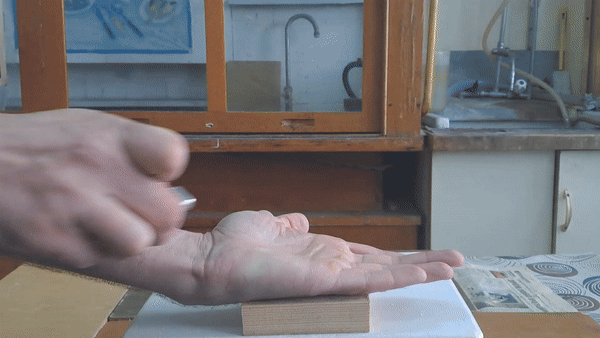
In front of my guests, I placed 0.2-0.3 g of acetone peroxide on my palm and ignited it. A bright yellow flash appeared, accompanied by a loud bang and the formation of soot. The substance burned quickly but did not damage my skin. The demonstration was well received; however, the initial video recording was poor, so I repeated the experiment several times from different angles to improve the footage. |

Fire on Hand: The Combustion of Acetone Peroxide |

|

|

|

|

|

|
|
|

|

|