Chemistry and Chemists № 2 2025
Journal of Chemists-Enthusiasts
| Content | Chemistry experiments - video | Physics experiments - video | Home Page - Chemistry and Chemists |
|
Chemistry and Chemists № 2 2025 Journal of Chemists-Enthusiasts |
Polytetrafluoroethylene (PTFE), Perfluoroalkoxy alkane (PFA), and Fluorinated ethylene propylene (FEP) Chemist |
|
Having noticed a mistake in the text, allocate it and press Ctrl-Enter
Polytetrafluoroethylene (PTFE), Perfluoroalkoxy alkane (PFA), and Fluorinated ethylene propylene (FEP)
A few months ago, I watched a video about a superacid composed of antimony pentafluoride and anhydrous hydrogen fluoride (HF + SbF5). The video claimed that this superacid could even dissolve wax candles! It was stored in a bottle made of a transparent polymer. Given the extreme chemical resistance required, I initially assumed the container was made of polytetrafluoroethylene (PTFE), a material well known for its high resistance to chemicals.
Политетрафторэтилен (PTFE) перфторалкоксиалкан (PFA) и фторированный этилен-пропилен (FEP) As its name suggests, polytetrafluoroethylene (PTFE) is a polymer of tetrafluoroethylene, (-CF2-CF2-)n. Due to its excellent chemical resistance, mechanical strength, and electrical insulation properties, PTFE is widely used in chemical laboratories and industry. However, PTFE products are typically white and opaque, whereas the container in the video was transparent, resembling polyethylene. After several frustrating attempts to understand the video (and cursing my poor English), I finally realized that the bottle was made of perfluoroalkoxy alkane (PFA). This material is a copolymer of tetrafluoroethylene and perfluorinated ethers, C2F3OR, where R is a perfluorinated alkyl radical, the simplest being -CF3. 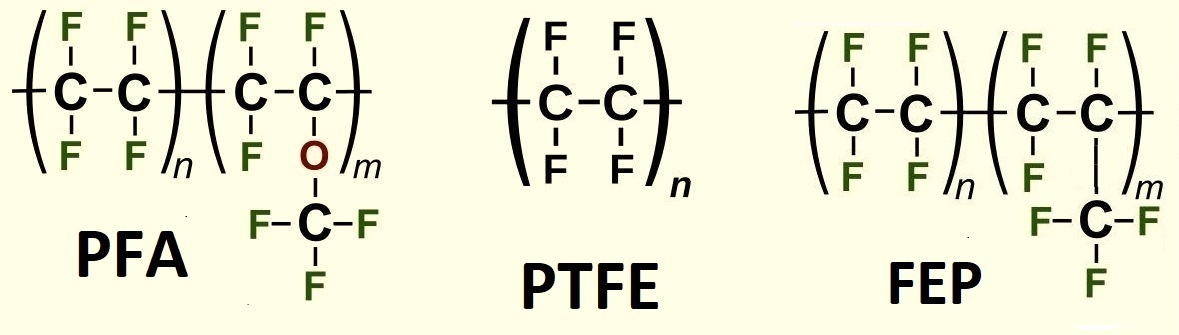
PFA shares many properties with PTFE, but there are key differences. For instance, PFA has even higher chemical resistance than PTFE. However, PTFE is more scratch-resistant and less expensive. Another significant difference lies in their processing methods. PFA, like polyethylene, polypropylene, and polystyrene, is a thermoplastic that can be molded in its molten state to form various products. Thermoplastics are characterized by their ability to transition into a fluid state upon heating without decomposition, which makes them much easier to process. Formally, PTFE is also classified as a thermoplastic. However, unlike other thermoplastics, PTFE cannot be processed via conventional melt processing methods. This is because PTFE begins to decompose at temperatures far below its melting point and has an extremely high melt viscosity. As a result, PTFE is processed using techniques more typical of thermosetting polymers - materials that undergo irreversible chemical transformations upon heating, preventing them from becoming fluid. Examples of thermosetting polymers include phenol-formaldehyde and epoxy resins. Unlike true thermosets, PTFE pieces can be welded together when heated, but this process is complex. It requires a vacuum chamber and temperatures close to PTFE's decomposition threshold. In my laboratory work, I frequently use PTFE products, but I had never encountered PFA items before and never expected to. That would have remained the case, but a colleague of mine has a habit of collecting all kinds of laboratory-related items - glassware, reagents, and equipment. He rarely uses them in his work, yet he has amassed an impressive stockpile of chemistry-related things. Recently, my colleague proudly announced that he had bought "transparent PTFE" and showed me a transparent tube. I had seen PTFE tubes many times before, but they were always white and opaque. However, this tube was completely colorless and transparent. I immediately suspected that the tube was not made of PTFE but rather of PFA. My colleague decided to verify this experimentally. He cut a piece from a white PTFE tube and secured it in place. Next to it, he fixed a piece of the transparent tube, which was likely made of PFA. He then directed a burner flame at both samples while attempting to stretch them with tweezers. The transparent tube softened and could be easily stretched - typical behavior for thermoplastic polymers, including PFA. The white PTFE tube turned transparent when heated but remained difficult to stretch. When exposed to stronger heating, the end of the tube began to char, exhibiting behavior characteristic of PTFE. Neither sample ignited in the flame. This experiment confirmed that the transparent tube was not made of PTFE but rather of PFA or another similar perfluorinated polymer. Later, I came across literature describing another polymer similar to PTFE and PFA that has gained widespread use - fluorinated ethylene propylene (FEP). This material is a copolymer of tetrafluoroethylene and hexafluoropropylene. Like PFA, FEP is transparent and colorless and can be melt-processed. However, it has lower chemical resistance compared to PTFE. |

Polytetrafluoroethylene (PTFE), Perfluoroalkoxy alkane (PFA), and Fluorinated ethylene propylene (FEP) |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

PTFE labware |

|

|

|

|